Abstract
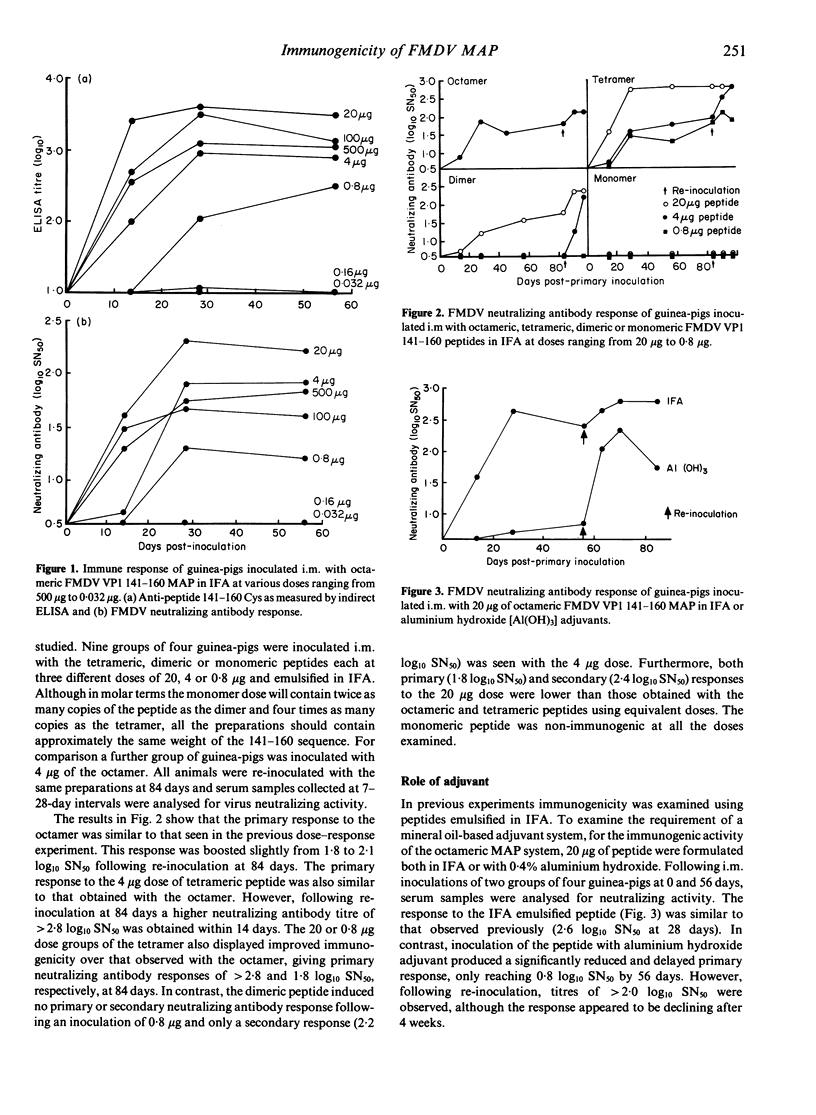
The multiple antigenic peptide (MAP) system for presenting epitopes to the immune system has been studied with an immunogenic foot-and-mouth disease virus (FMDV) peptide comprising amino acids 141-160 of protein VP1. Neutralizing antibody responses known to protect guinea-pigs against challenge infection were obtained with a single inoculation of 0.8-4 micrograms of peptide, presented as an octamer or a tetramer, whereas 20 micrograms of a dimer were required to evoke a similar level of antibody. A monomeric preparation did not elicit measurable levels of neutralizing antibody at doses up to 20 micrograms. The octameric MAP was also immunogenic using an aluminum hydroxide adjuvant. Antibodies elicited by the octameric, tetrameric and dimeric constructs differed qualitatively in their reaction with sequences within the 141-160 peptide. Those against the octamer reacted poorly with peptides within the 141-160 sequence, whereas those elicited by the tetramer and dimer reacted preferentially with the peptides covering the N-terminal region. The levels of neutralizing antibody obtained with the octamer and tetramer compare favourably with those obtained when the FMDV peptide is attached to carrier proteins but are lower than those obtained when it is presented as part of a peptide-hepatitis B virus core particle. Nevertheless, the ability to elicit protective levels of neutralizing antibody without the use of a carrier protein would be a distinct advantage in the development of synthetic peptide vaccines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Broekhuijsen M. P., van Rijn J. M., Blom A. J., Pouwels P. H., Enger-Valk B. E., Brown F., Francis M. J. Fusion proteins with multiple copies of the major antigenic determinant of foot-and-mouth disease virus protect both the natural host and laboratory animals. J Gen Virol. 1987 Dec;68(Pt 12):3137–3143. doi: 10.1099/0022-1317-68-12-3137. [DOI] [PubMed] [Google Scholar]
- Clarke B. E., Brown A. L., Grace K. G., Hastings G. Z., Brown F., Rowlands D. J., Francis M. J. Presentation and immunogenicity of viral epitopes on the surface of hybrid hepatitis B virus core particles produced in bacteria. J Gen Virol. 1990 May;71(Pt 5):1109–1117. doi: 10.1099/0022-1317-71-5-1109. [DOI] [PubMed] [Google Scholar]
- Clarke B. E., Newton S. E., Carroll A. R., Francis M. J., Appleyard G., Syred A. D., Highfield P. E., Rowlands D. J., Brown F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. 1987 Nov 26-Dec 2Nature. 330(6146):381–384. doi: 10.1038/330381a0. [DOI] [PubMed] [Google Scholar]
- DiMarchi R., Brooke G., Gale C., Cracknell V., Doel T., Mowat N. Protection of cattle against foot-and-mouth disease by a synthetic peptide. Science. 1986 May 2;232(4750):639–641. doi: 10.1126/science.3008333. [DOI] [PubMed] [Google Scholar]
- Fox G., Parry N. R., Barnett P. V., McGinn B., Rowlands D. J., Brown F. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J Gen Virol. 1989 Mar;70(Pt 3):625–637. doi: 10.1099/0022-1317-70-3-625. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Black L. Antibody response in pig nasal fluid and serum following foot-and-mouth disease infection or vaccination. J Hyg (Lond) 1983 Oct;91(2):329–334. doi: 10.1017/s0022172400060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M. J., Clarke B. E. Peptide vaccines based on enhanced immunogenicity of peptide epitopes presented with T-cell determinants or hepatitis B core protein. Methods Enzymol. 1989;178:659–676. doi: 10.1016/0076-6879(89)78044-7. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Fry C. M., Rowlands D. J., Bittle J. L., Houghten R. A., Lerner R. A., Brown F. Immune response to uncoupled peptides of foot-and-mouth disease virus. Immunology. 1987 May;61(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Francis M. J., Fry C. M., Rowlands D. J., Brown F., Bittle J. L., Houghten R. A., Lerner R. A. Immunological priming with synthetic peptides of foot-and-mouth disease virus. J Gen Virol. 1985 Nov;66(Pt 11):2347–2354. doi: 10.1099/0022-1317-66-11-2347. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Fry C. M., Rowlands D. J., Brown F. Qualitative and quantitative differences in the immune response to foot-and-mouth disease virus antigens and synthetic peptides. J Gen Virol. 1988 Oct;69(Pt 10):2483–2491. doi: 10.1099/0022-1317-69-10-2483. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Hastings G. Z., Clarke B. E., Brown A. L., Beddell C. R., Rowlands D. J., Brown F. Neutralizing antibodies to all seven serotypes of foot-and-mouth disease virus elicited by synthetic peptides. Immunology. 1990 Feb;69(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Francis M. J. Peptide vaccines for viral diseases. Sci Prog. 1990;74(293 Pt 1):115–130. [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Moriarty A., Thornton G. B. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987 Aug 15;139(4):1223–1231. [PubMed] [Google Scholar]
- Murdin A. D., Doel T. R. Synthetic peptide vaccines against foot-and-mouth disease. II. Comparison of the response of guinea-pigs, rabbits and mice to various formulations. J Biol Stand. 1987 Jan;15(1):53–65. doi: 10.1016/0092-1157(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Mussgay M., Böhm H. O., Schulz G. E., Schaller H. Antibodies against a preselected peptide recognize and neutralize foot and mouth disease virus. EMBO J. 1982;1(7):869–874. doi: 10.1002/j.1460-2075.1982.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N., McGrath H., Tam J. P. A novel method for producing anti-peptide antibodies. Production of site-specific antibodies to the T cell antigen receptor beta-chain. J Biol Chem. 1988 Feb 5;263(4):1719–1725. [PubMed] [Google Scholar]
- Tam J. P., Lu Y. A. Vaccine engineering: enhancement of immunogenicity of synthetic peptide vaccines related to hepatitis in chemically defined models consisting of T- and B-cell epitopes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9084–9088. doi: 10.1073/pnas.86.23.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P., Zavala F. Multiple antigen peptide. A novel approach to increase detection sensitivity of synthetic peptides in solid-phase immunoassays. J Immunol Methods. 1989 Nov 13;124(1):53–61. doi: 10.1016/0022-1759(89)90185-3. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E. Enzyme-immunoassays for antibodies in measles, cytomegalovirus infections and after rubella vaccination. Br J Exp Pathol. 1976 Apr;57(2):243–247. [PMC free article] [PubMed] [Google Scholar]



