Abstract
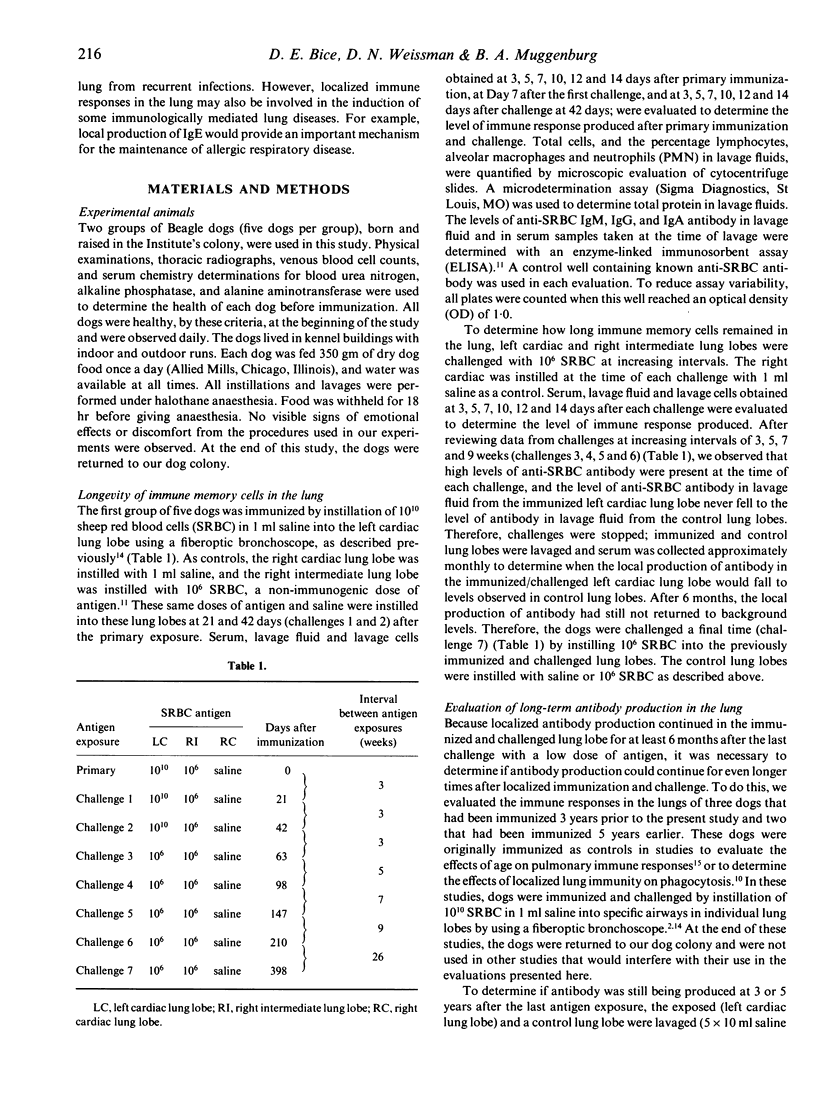
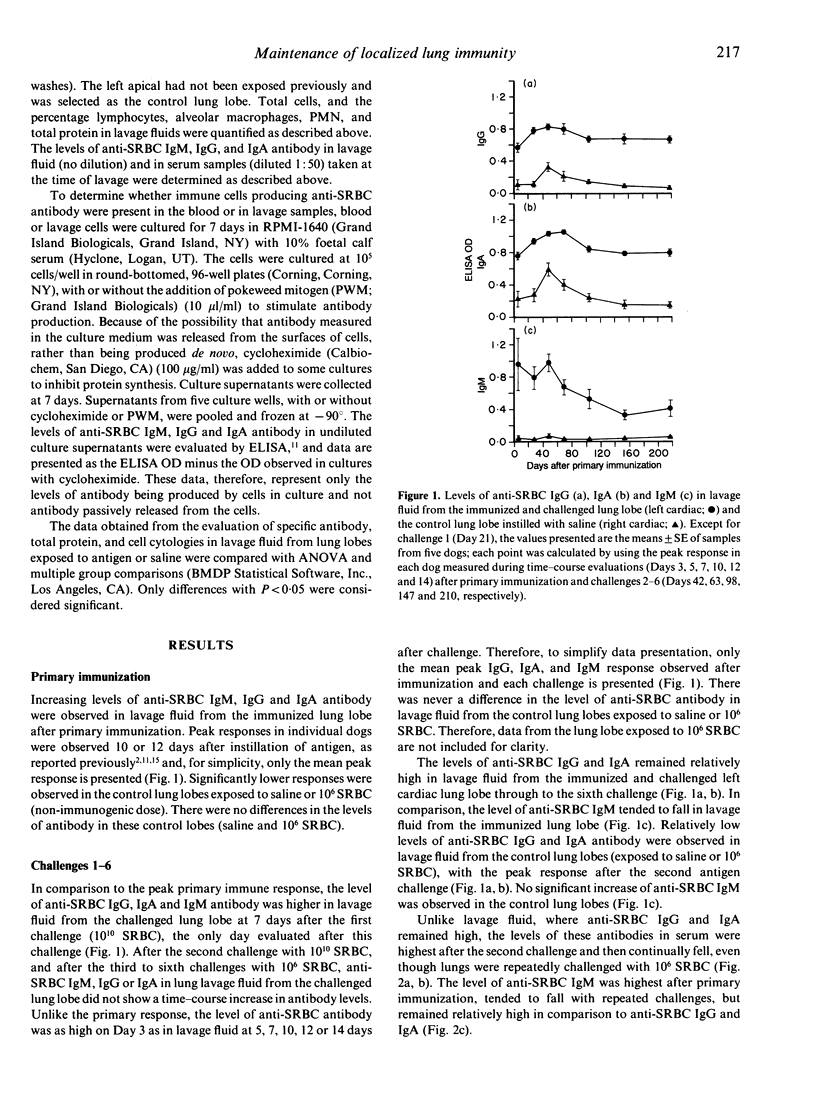
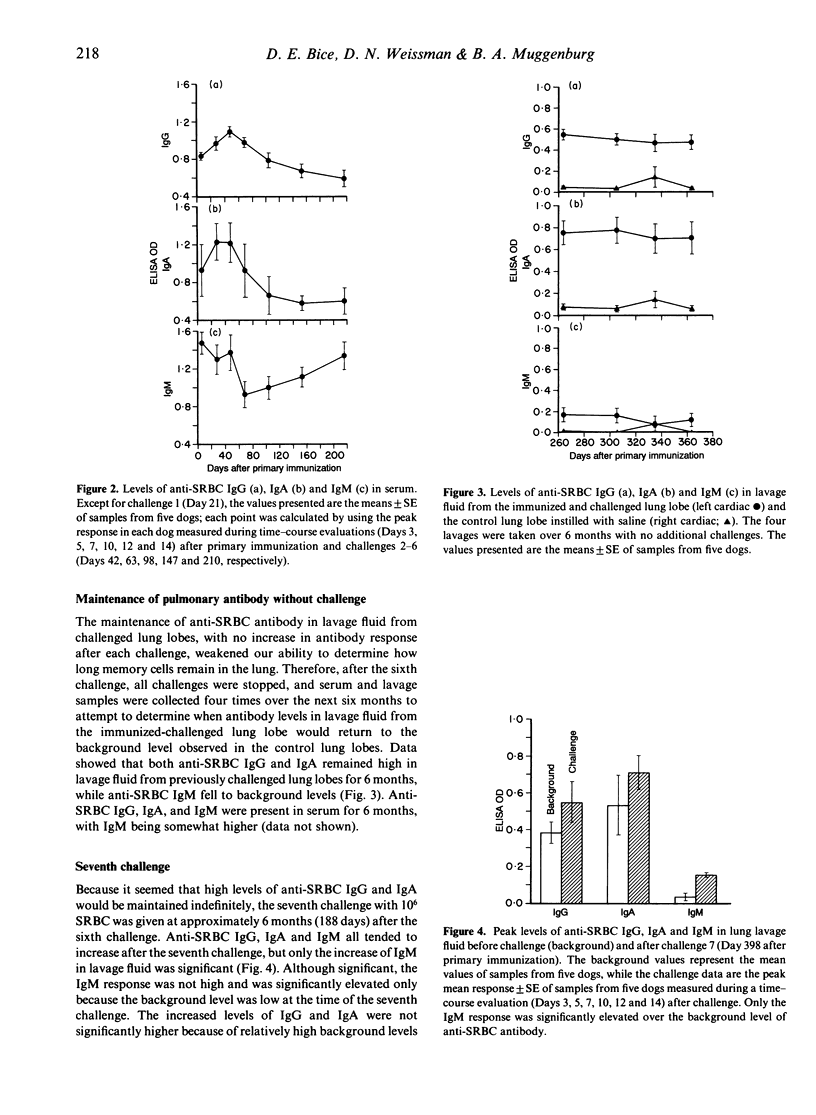
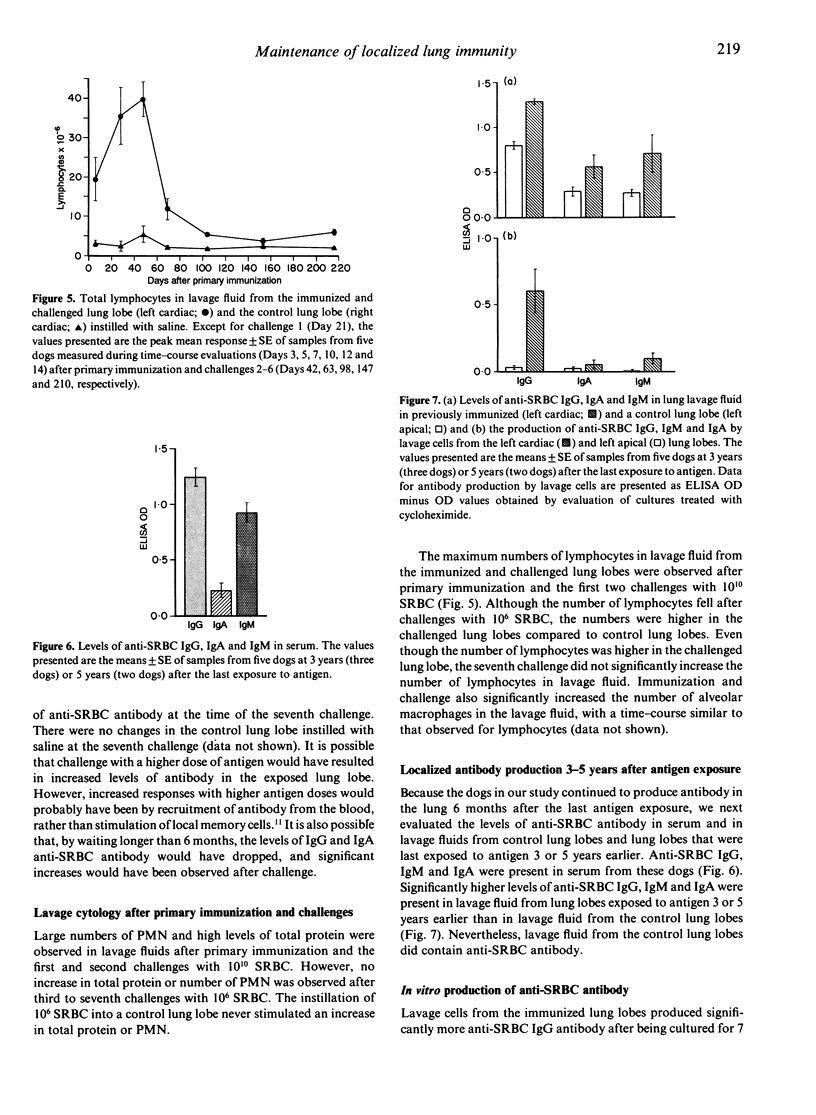
Immune memory cells for antibody production in the lung respond to antigen challenges, providing an important pulmonary defence. However, studies reported here suggested that long-term antibody production in the lung might also be important in pulmonary immune memory. After localized lung immunization and challenges, antibody production of specific IgG and IgA continued in the immunized lung lobes of dogs for months after the last antigen exposure. In addition, the evaluation of lung immunity in dogs immunized and challenged 3 years or 5 years previously (without additional antigen challenge) showed significantly higher levels of specific antibody in lavage fluid from the lung lobe exposed to antigen than in lavage fluids from control lung lobes. Cells from blood or from control lung lobes did not produce significant levels of specific antibody in vitro, whereas cells lavaged from the immunized lung lobes were producing specific antibody. Therefore, long-term antibody production by cells in lung lobes exposed to antigen probably contributed to antibody levels in serum and unexposed lung lobes. Traditionally, lymphoid tissues are believed to be responsible for long-term antibody production. However, antibody production in the lung for years without repeated antigen exposure suggested that other tissues might also be important in long-term antibody production. Maintenance of localized antibody production in the lung would be an important pulmonary defence against infectious agents, but might also play a key role in hypersensitivity lung diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansfield M. J., Kaltreider H. B., Caldwell J. L., Herskowitz F. N. Hyporesponsiveness of canine bronchoalveolar lymphocytes to mitogens: inhibition of lymphocyte proliferation by alveolar macrophages. J Immunol. 1979 Feb;122(2):542–548. [PubMed] [Google Scholar]
- Bice D. E., Degen M. A., Harris D. L., Muggenburg B. A. Recruitment of antibody-forming cells in the lung after local immunization is nonspecific. Am Rev Respir Dis. 1982 Oct;126(4):635–639. doi: 10.1164/arrd.1982.126.4.635. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Gray R. H., Evans M. J., Muggenburg B. A. Identification of plasma cells in lung alveoli and interstitial tissues after localized lung immunization. J Leukoc Biol. 1987 Jan;41(1):1–7. doi: 10.1002/jlb.41.1.1. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Harris D. L., Hill J. O., Muggenburg B. A., Wolff R. K. Immune responses after localized lung immunization in the dog. Am Rev Respir Dis. 1980 Nov;122(5):755–760. doi: 10.1164/arrd.1980.122.5.755. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Harris D. L., Muggenburg B. A. Regional immunologic responses following localized deposition of antigen in the lung. Exp Lung Res. 1980 Mar;1(1):33–41. doi: 10.3109/01902148009057511. [DOI] [PubMed] [Google Scholar]
- Bice D. E., King-Herbert A. P., Morris M. J., Hanna N., Haley P. J. Inflammation and recruitment of immune cells into the lung. Reg Immunol. 1989 Nov-Dec;2(6):376–384. [PubMed] [Google Scholar]
- Bice D. E., Muggenburg B. A. Effect of age on antibody responses after lung immunization. Am Rev Respir Dis. 1985 Sep;132(3):661–665. doi: 10.1164/arrd.1985.132.3.661. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Muggenburg B. A. Localized immune memory in the lung. Am Rev Respir Dis. 1988 Sep;138(3):565–571. doi: 10.1164/ajrccm/138.3.565. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Shopp G. M. Antibody responses after lung immunization. Exp Lung Res. 1988;14(2):133–155. doi: 10.3109/01902148809115121. [DOI] [PubMed] [Google Scholar]
- Couch R. B., Kasel J. A. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- Demenkoff J. H., Ansfield M. J., Kaltreider H. B., Adam E. Alveolar macrophage suppression of canine bronchoalveolar lymphocytes: the role of prostaglandin E2 in the inhibition of mitogen-responses. J Immunol. 1980 Mar;124(3):1365–1370. [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. Role of antigen in migration patterns of T cell subsets arising from gut-associated lymphoid tissue. Reg Immunol. 1989 Jul-Aug;2(4):213–224. [PubMed] [Google Scholar]
- Glezen W. P., Couch R. B., Six H. R. The influenza herald wave. Am J Epidemiol. 1982 Oct;116(4):589–598. doi: 10.1093/oxfordjournals.aje.a113441. [DOI] [PubMed] [Google Scholar]
- Harmsen A. G., Bice D. E., Muggenburg B. A. The effect of local antibody responses on in vivo and in vitro phagocytosis by pulmonary alveolar macrophages. J Leukoc Biol. 1985 May;37(5):483–492. doi: 10.1002/jlb.37.5.483. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Sedgwick J. D., O'Leary C., Krska K., Leivers S. Long-lived IgE- and IgG-secreting cells in rodents manifesting persistent antibody responses. Cell Immunol. 1984 Dec;89(2):281–289. doi: 10.1016/0008-8749(84)90330-7. [DOI] [PubMed] [Google Scholar]
- Jones P. D., Ada G. L. Influenza-specific antibody-secreting cells and B cell memory in the murine lung after immunization with wild-type, cold-adapted variant and inactivated influenza viruses. Vaccine. 1987 Sep;5(3):244–248. doi: 10.1016/0264-410x(87)90109-5. [DOI] [PubMed] [Google Scholar]
- Jones P. D., Ada G. L. Persistence of influenza virus-specific antibody-secreting cells and B-cell memory after primary murine influenza virus infection. Cell Immunol. 1987 Oct 1;109(1):53–64. doi: 10.1016/0008-8749(87)90291-7. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Barth E., Pellegrini C. The effect of splenectomy on the appearance of specific antibody-forming cells in lungs of dogs after intravenous immunization with sheep erythrocytes. Exp Lung Res. 1981 Aug;2(3):231–238. doi: 10.3109/01902148109052318. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Caldwell J. L., Adam E. The fate and consequence of an organic particulate antigen instilled into bronchoalveolar spaces of normal canine lungs. Am Rev Respir Dis. 1977 Aug;116(2):267–280. doi: 10.1164/arrd.1977.116.2.267. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Kyselka L., Salmon S. E. Immunology of the lower respiratory tract. II. The plaque-forming response of canine lymphoid tissues to sheep erythrocytes after intrapulmonary or intravenous immunization. J Clin Invest. 1974 Aug;54(2):263–270. doi: 10.1172/JCI107761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb M. F., Toews G. B., Lyons C. R., Uhr J. W. Antigen presentation by guinea pig alveolar macrophages. J Immunol. 1981 Jan;126(1):286–291. [PubMed] [Google Scholar]
- MILLER J. J., 3rd AN AUTORADIOGRAPHIC STUDY OF PLASMA CELL AND LYMPHOCYTE SURVIVAL IN RAT POPLITEAL LYMPH NODES. J Immunol. 1964 May;92:673–681. [PubMed] [Google Scholar]
- Mandel T. E., Phipps R. P., Abbot A., Tew J. G. The follicular dendritic cell: long term antigen retention during immunity. Immunol Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Bice D. E., Muggenburg B. A. Local pulmonary immune responsiveness after multiple antigenic exposures in the cynomolgus monkey. Am Rev Respir Dis. 1985 Sep;132(3):657–660. doi: 10.1164/arrd.1985.132.3.657. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Gillett N. A., Bice D. E. Comparison of systemic and local immune responses after multiple pulmonary antigen exposures. Reg Immunol. 1989 May-Jun;2(3):149–157. [PubMed] [Google Scholar]
- Nicod L. P., Lipscomb M. F., Weissler J. C., Lyons C. R., Albertson J., Toews G. B. Mononuclear cells in human lung parenchyma. Characterization of a potent accessory cell not obtained by bronchoalveolar lavage. Am Rev Respir Dis. 1987 Oct;136(4):818–823. doi: 10.1164/ajrccm/136.4.818. [DOI] [PubMed] [Google Scholar]
- Peeters S. H., Carter B. G. Regulation of the IgE antibody response in mice. I. Long-term production of IgE Anti-ovalbumin antibody in irradiated recipients. J Immunol. 1978 Oct;121(4):1596–1602. [PubMed] [Google Scholar]
- Peeters S. H., Carter B. G. Regulation of the IgE antibody response in mice. II. Radioresistance of established IgE antibody production. Immunology. 1981 May;43(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- Rochester C. L., Goodell E. M., Stoltenborg J. K., Bowers W. E. Dendritic cells from rat lung are potent accessory cells. Am Rev Respir Dis. 1988 Jul;138(1):121–128. doi: 10.1164/ajrccm/138.1.121. [DOI] [PubMed] [Google Scholar]
- Schuyler M. R., Todd L. S. Accessory cell function of rabbit alveolar macrophages. Am Rev Respir Dis. 1981 Jan;123(1):53–57. doi: 10.1164/arrd.1981.123.1.53. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Suppression of IgE responses in inbred rats by repeated respiratory tract exposure to antigen: responder phenotype influences isotype specificity of induced tolerance. Eur J Immunol. 1984 Oct;14(10):893–897. doi: 10.1002/eji.1830141006. [DOI] [PubMed] [Google Scholar]
- Soler P., Moreau A., Basset F., Hance A. J. Cigarette smoking-induced changes in the number and differentiated state of pulmonary dendritic cells/Langerhans cells. Am Rev Respir Dis. 1989 May;139(5):1112–1117. doi: 10.1164/ajrccm/139.5.1112. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Phipps R. P., Mandel T. E. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Thorbecke G. J., Steinman R. M. Dendritic cells in the immune response: characteristics and recommended nomenclature (A report from the Reticuloendothelial Society Committee on Nomenclature). J Reticuloendothel Soc. 1982 May;31(5):371–380. [PubMed] [Google Scholar]



