Abstract
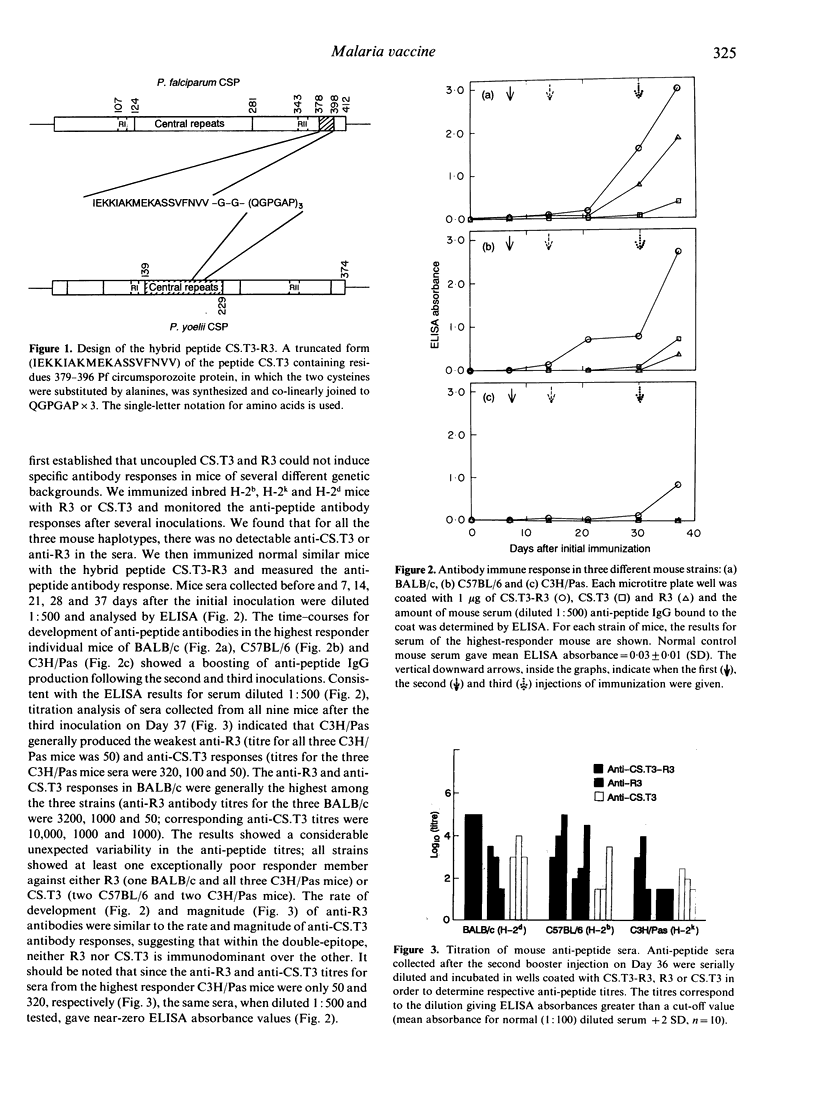
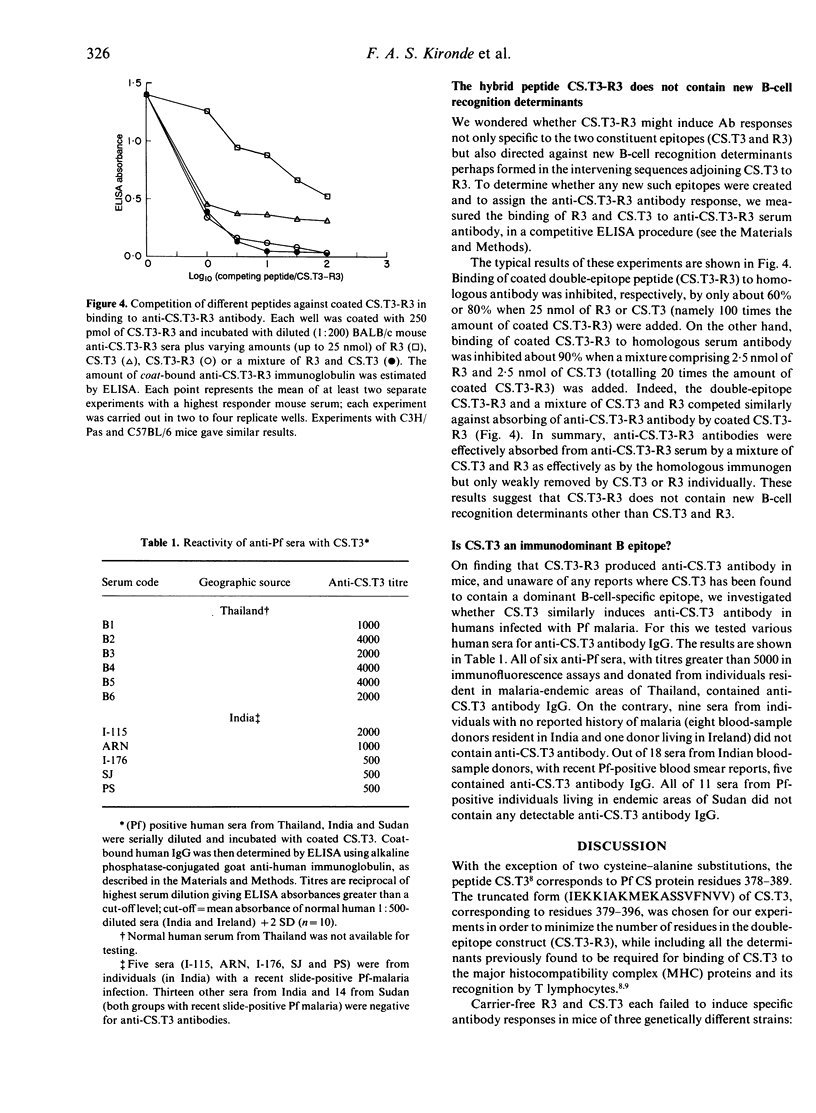
The peptide CS.T3, corresponding to residues 378-398 of the Plasmodium falciparum (Pf) circumsporozoite (CS) protein sequence (except with cysteines 384 and 389 replaced by alanines), has been found to be almost universally recognized by human and mouse T lymphocytes. When colinearly linked to the repetitive B-lymphocyte-specific epitope (Asn-Ala-Asn-Pro)n of Pf CS protein, CS.T3 induces T-helper activity for an anti-(Asn-Ala-Asn-Pro)n antibody response in mice of different haplotypes. We constructed a double-epitope peptide, CS.T3-R3, by co-linearly joining a truncated 18-mer form (IEKKIAKMEKASSVFNVV) of CS.T3 to three tandem repeats (R3) of a B-cell-specific epitope, QGPGAP, of Plasmodium yoelii (Py) CS protein, via a two-glycine spacer. Whereas CS.T3 and R3 did not induce specific antibodies, CS.T3-R3 elicited anti-CS.T3 and anti-R3 antibodies in different mouse strains. Some human anti-Pf sera from malaria-endemic areas contained high-titred anti-CS.T3 antibody IgG, indicating that parasite-derived CS.T3 contains a B-cell determinant which is maintained in the alanine-substituted synthetic CS.T3. Antibody absorption experiments showed that CS.T3-R3 contains no new B-cell-specific determinants other than R3 and CS.T3. That the Pf CS protein epitope, CS.T3, supports T-cell help for antibody responses against the Py CS protein repeat epitope, QGPGAP, implies the possible use of CS.T3 in anti-sporozoite multiple-epitope vaccines against different species of Plasmodium. Colinearly linking CS.T3 to R3, via a two-glycine spacer, appears to be a useful model by which different T- and B-cell-specific determinants can be jointed into a heterovalent immunogen while retaining their distinct immunological properties.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter R., Kumar N., Quakyi I., Good M., Mendis K., Graves P., Miller L. Immunity to sexual stages of malaria parasites. Prog Allergy. 1988;41:193–214. [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Gillessen D., Lahm H. W., Matile H., Schönfeld H. J., Trzeciak A. Use of prior vaccinations for the development of new vaccines. Science. 1990 Jul 27;249(4967):423–425. doi: 10.1126/science.1696030. [DOI] [PubMed] [Google Scholar]
- Golvano J., Lasarte J. J., Sarobe P., Gullón A., Prieto J., Borrás-Cuesta F. Polarity of immunogens: implications for vaccine design. Eur J Immunol. 1990 Oct;20(10):2363–2366. doi: 10.1002/eji.1830201031. [DOI] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley T. J., Miller L. H. Invasion of erythrocytes by malaria parasites: erythrocyte ligands and parasite receptors. Prog Allergy. 1988;41:49–71. [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F., Walliker D. Genetic diversity in Plasmodium falciparum. Adv Parasitol. 1990;29:75–149. doi: 10.1016/s0065-308x(08)60105-0. [DOI] [PubMed] [Google Scholar]
- Lal A. A., de la Cruz V. F., Good M. F., Weiss W. R., Lunde M., Maloy W. L., Welsh J. A., McCutchan T. F. In vivo testing of subunit vaccines against malaria sporozoites using a rodent system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8647–8651. doi: 10.1073/pnas.84.23.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardin E. H., Barr P. J., Heimer E., Etlinger H. M. Genetic restriction of the murine humoral response to a recombinant Plasmodium vivax circumsporozoite protein. Eur J Immunol. 1988 Jul;18(7):1119–1122. doi: 10.1002/eji.1830180722. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L. A., Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988 Mar 10;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- Rao K. V., Nayak A. R. Enhanced immunogenicity of a sequence derived from hepatitis B virus surface antigen in a composite peptide that includes the immunostimulatory region from human interleukin 1. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5519–5522. doi: 10.1073/pnas.87.14.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Gillessen D., Doran D. M., Takacs B., Matile H., Trzeciak A., Pink J. R. Epitopes recognized by human T lymphocytes on malaria circumsporozoite protein. Eur J Immunol. 1988 Apr;18(4):633–636. doi: 10.1002/eji.1830180422. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]