Abstract
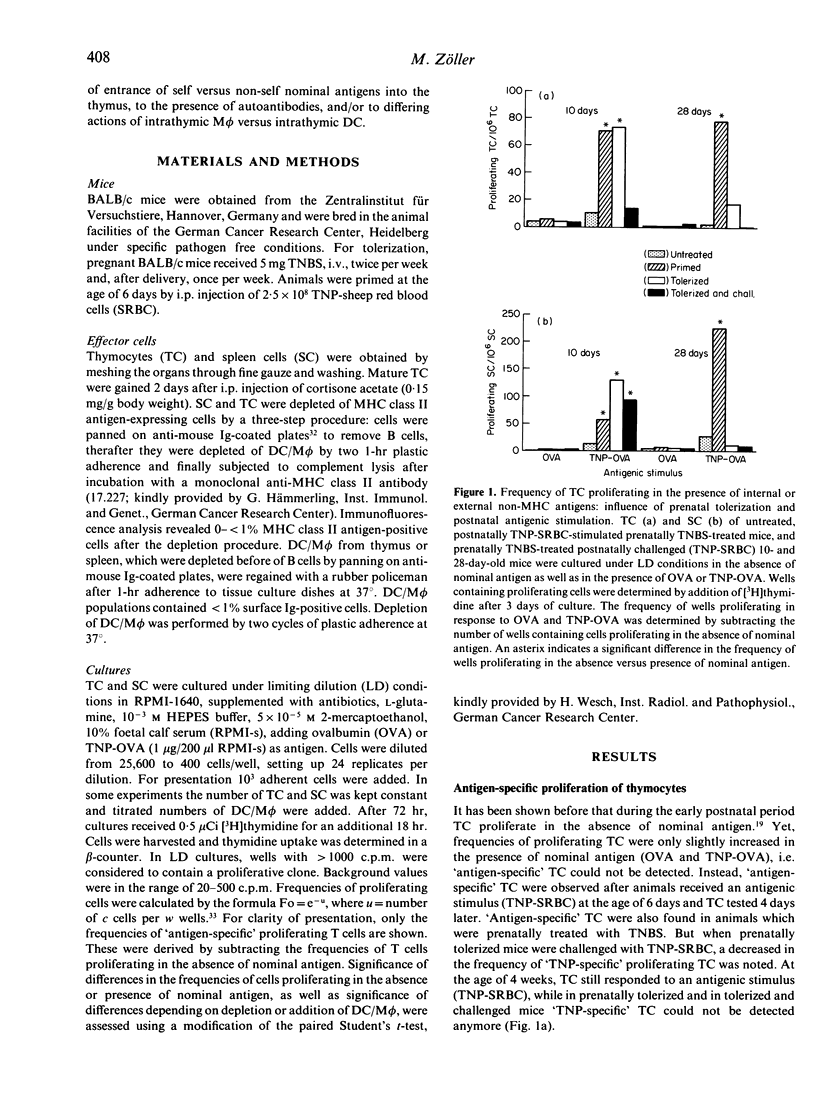
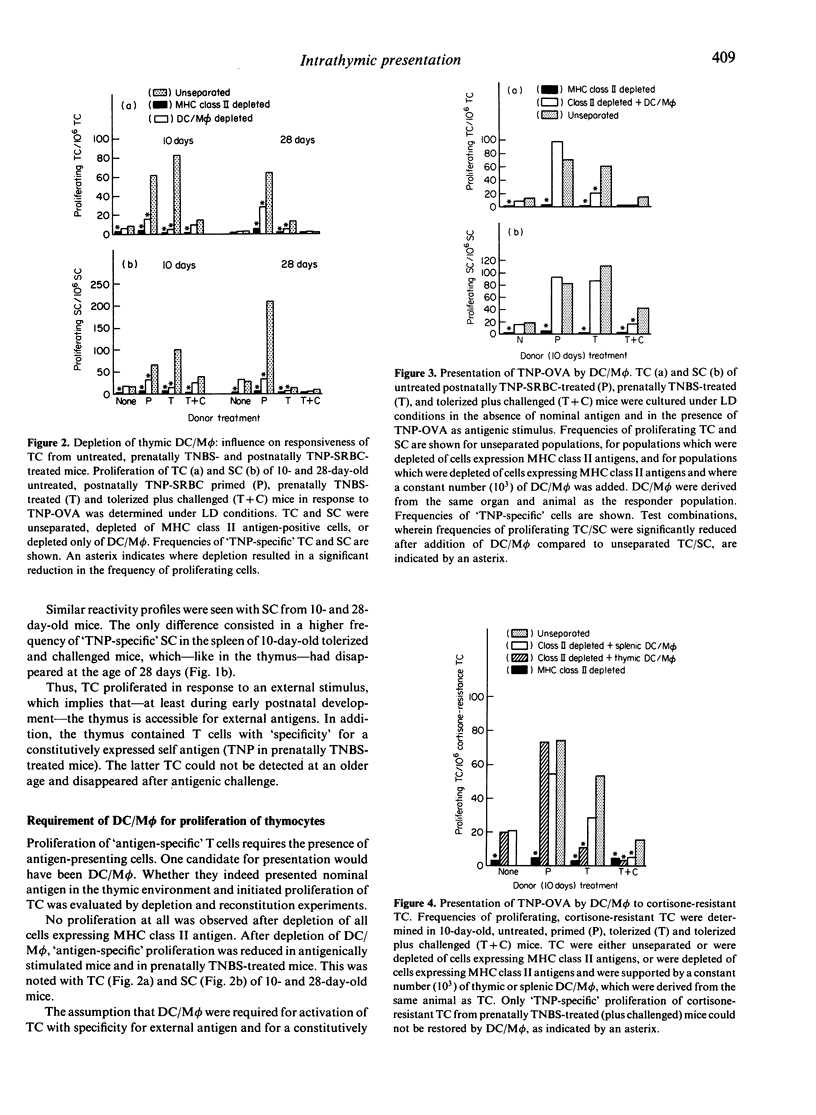
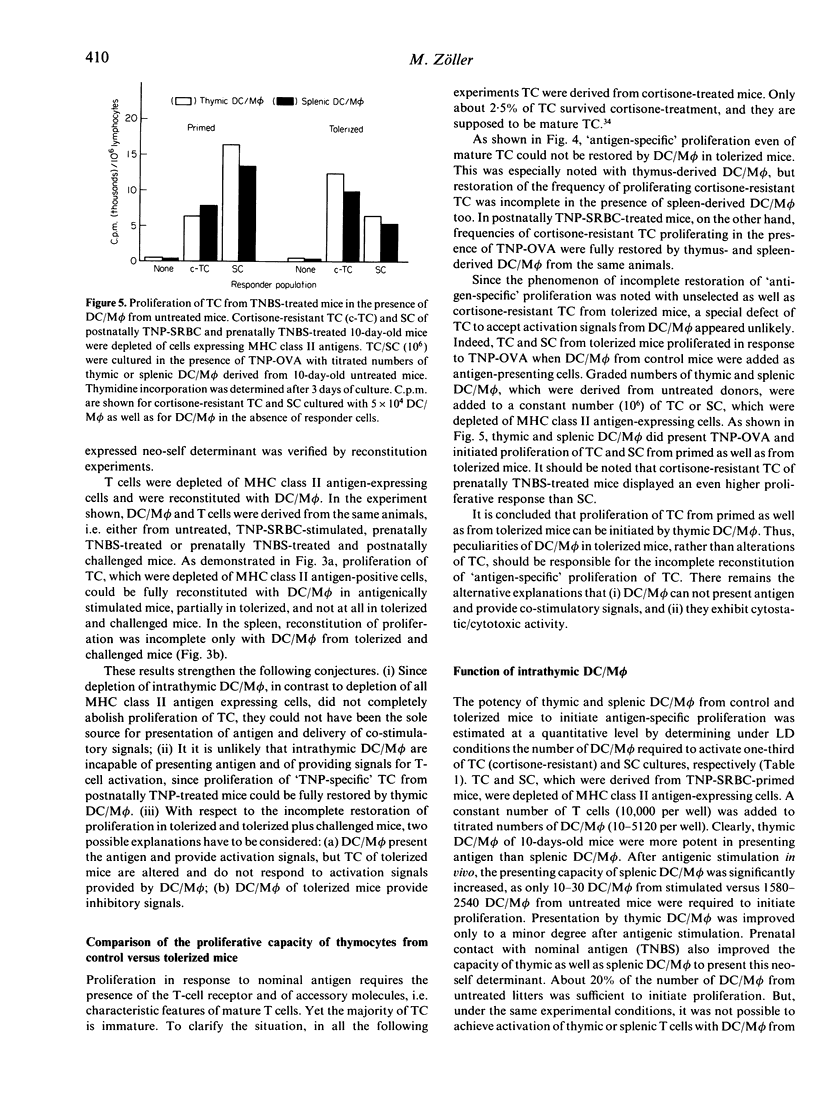
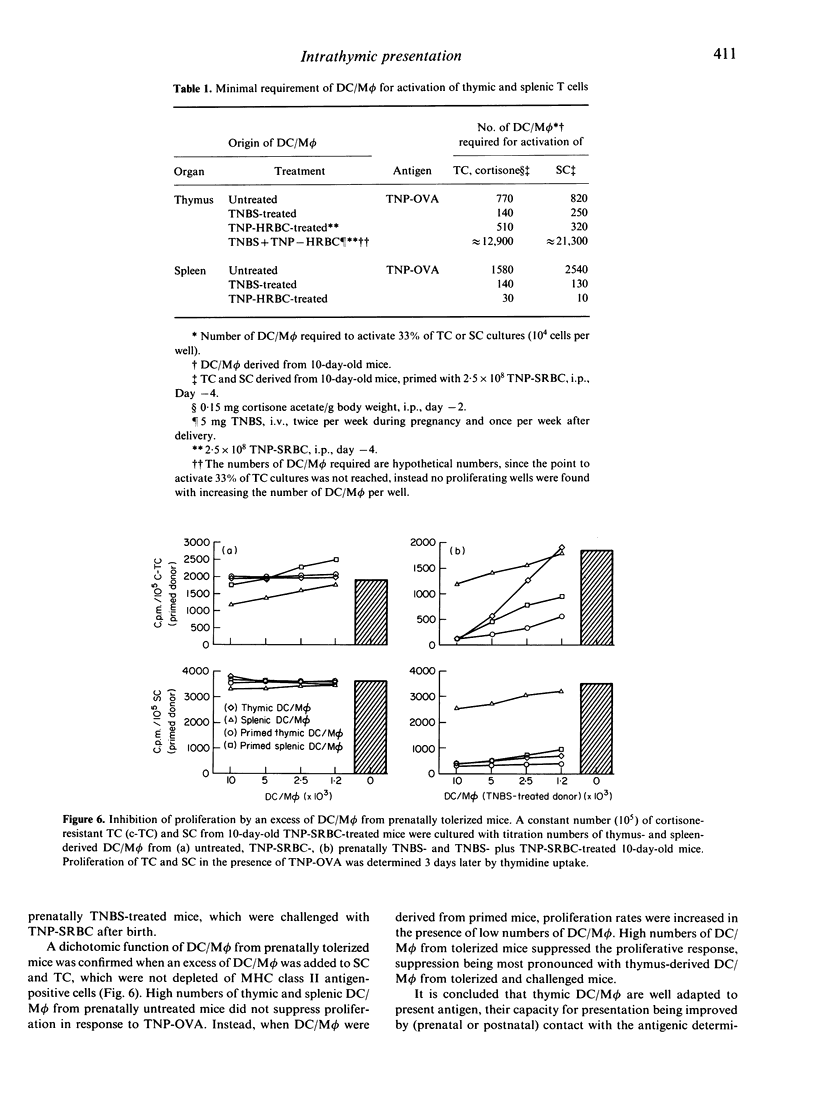
The present study focused on the question of whether intrathymic dendritic cells and macrophages (DC/M phi) are involved in the processes of T-cell repertoire selection and establishment of tolerance towards nominal antigen. Proliferation of thymocytes (TC) was determined under limiting dilution (LD) conditions after depletion of DC/M phi and after reconstitution of TC, which were depleted of cells expressing major histocompatibility complex (MHC) class II antigens, with thymic DC/M phi. Trinitrophenyl (TNP) [coupled to ovalbumin (OVA)] was used as an internal antigen in prenatally trinitrobenzenesulphonic acid (TNBS)-treated mice and as an external antigen in prenatally untreated mice. Intrathymic DC/M phi were clearly involved in selecting the repertoire of T cells specific for external antigen: they presented the antigen and initiated proliferation of thymic T cells, which were depleted of MHC class II antigen-expressing cells. But they were not the only cells to present nominal antigen in the thymic environment. Intrathymic DC/M phi could also deliver negative signals. This became apparent when evaluating presentation of TNP in prenatally TNBS-treated mice. Thymus-derived DC/M phi from prenatally TNBS-treated mice could not initiate proliferation of TC in response to TNP-OVA. Instead, when prenatally TNBS-treated mice received an antigenic challenge [TNP-sheep red blood cells (SRBC)], thymic DC/M phi inhibited proliferation of cortisone-resistant TC from untreated and prenatally TNBS-treated mice. This can be explained by assuming that in the process of establishing tolerance, intrathymic DC/M phi may exert cytotoxic/cytostatic activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtnich M., Zöller M. Autoreactive antibodies in thymus and spleen of neonatal and young adult BALB/c mice: influence of prenatal tolerization. Scand J Immunol. 1991 Jan;33(1):25–36. doi: 10.1111/j.1365-3083.1991.tb02489.x. [DOI] [PubMed] [Google Scholar]
- Blackman M. A., Kappler J. W., Marrack P. T-cell specificity and repertoire. Immunol Rev. 1988 Jan;101:5–19. doi: 10.1111/j.1600-065x.1988.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- GALLILY R., WARWICK A., BANG F. B. EFFECT OF CORTISONE OF GENETIC RESISTANCE TO MOUSE HEPATITIS VIRUS IN VIVO AND IN VITRO. Proc Natl Acad Sci U S A. 1964 Jun;51:1158–1164. doi: 10.1073/pnas.51.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Kinashi T., Tashiro K., Witmer-Pack M., Crowley M., Kaplan G., Valinsky J., Romani N., Ikehara S. Macrophages phagocytose thymic lymphocytes with productively rearranged T cell receptor alpha and beta genes. J Exp Med. 1988 Dec 1;168(6):2279–2294. doi: 10.1084/jem.168.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson P. G., Norton A. J., Addis B. J. The human thymus contains a novel population of B lymphocytes. Lancet. 1987 Dec 26;2(8574):1488–1491. doi: 10.1016/s0140-6736(87)92622-5. [DOI] [PubMed] [Google Scholar]
- Iwata M., Hanaoka S., Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol. 1991 Mar;21(3):643–648. doi: 10.1002/eji.1830210316. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Ron J., Katz M. E. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987 Feb 15;138(4):1051–1055. [PubMed] [Google Scholar]
- Jenkinson E. J., Jhittay P., Kingston R., Owen J. J. Studies of the role of the thymic environment in the induction of tolerance to MHC antigens. Transplantation. 1985 Mar;39(3):331–333. doi: 10.1097/00007890-198503000-00030. [DOI] [PubMed] [Google Scholar]
- Kampinga J., Nieuwenhuis P., Roser B., Aspinall R. Differences in turnover between thymic medullary dendritic cells and a subset of cortical macrophages. J Immunol. 1990 Sep 15;145(6):1659–1663. [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kimoto H., Shirasawa T., Taniguchi M., Takemori T. B cell precursors are present in the thymus during early development. Eur J Immunol. 1989 Jan;19(1):97–104. doi: 10.1002/eji.1830190116. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Lafontaine M., Landry D., Blanc-Brunât N., Pelletier M., Montplaisir S. IL-1 production by human thymic dendritic cells: studies on the interrelation with DC accessory function. Cell Immunol. 1991 Jul;135(2):431–444. doi: 10.1016/0008-8749(91)90288-m. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Lorenz R. G., Allen P. M. Thymic cortical epithelial cells can present self-antigens in vivo. Nature. 1989 Feb 9;337(6207):560–562. doi: 10.1038/337560a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Pedrazzini T., Schneider R., Louis J. A., Zinkernagel R. M., Hengartner H. Intrathymic elimination of Mlsa-reactive (V beta 6+) cells during neonatal tolerance induction to Mlsa-encoded antigens. J Exp Med. 1988 Jun 1;167(6):2005–2010. doi: 10.1084/jem.167.6.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T-cell repertoire for antigen and MHC. Immunol Today. 1988 Oct;9(10):308–315. doi: 10.1016/0167-5699(88)91324-2. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Born W., McDuffie M., Kappler J. The development of helper T cell precursors in mouse thymus. J Immunol. 1988 Apr 15;140(8):2508–2514. [PubMed] [Google Scholar]
- Marrack P., Lo D., Brinster R., Palmiter R., Burkly L., Flavell R. H., Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988 May 20;53(4):627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989 Mar 2;338(6210):74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- Mazda O., Watanabe Y., Gyotoku J., Katsura Y. Requirement of dendritic cells and B cells in the clonal deletion of Mls-reactive T cells in the thymus. J Exp Med. 1991 Mar 1;173(3):539–547. doi: 10.1084/jem.173.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J. P., Puré E., Steinman R. M. Distinct features of dendritic cells and anti-Ig activated B cells as stimulators of the primary mixed leukocyte reaction. J Exp Med. 1989 Jan 1;169(1):239–254. doi: 10.1084/jem.169.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis P., Stet R. J., Wagenaar J. P., Wubbena A. S., Kampinga J., Karrenbeld A. The transcapsular route: a new way for (self-) antigens to by-pass the blood-thymus barrier? Immunol Today. 1988 Dec;9(12):372–375. doi: 10.1016/0167-5699(88)91236-4. [DOI] [PubMed] [Google Scholar]
- Ransom J., Wu R., Fischer M., Zlotnik A. Antigen presenting ability of thymic macrophages and epithelial cells: evidence for defects in the antigen processing function of thymic epithelial cells. Cell Immunol. 1991 Apr 15;134(1):180–190. doi: 10.1016/0008-8749(91)90341-8. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Sharrow S. O., Singer A. Clonal deletion and clonal anergy in the thymus induced by cellular elements with different radiation sensitivities. J Exp Med. 1990 Mar 1;171(3):935–940. doi: 10.1084/jem.171.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Schneider R., Lees R. K., Pedrazzini T., Zinkernagel R. M., Hengartner H., MacDonald H. R. Postnatal disappearance of self-reactive (V beta 6+) cells from the thymus of Mlsa mice. Implications for T cell development and autoimmunity. J Exp Med. 1989 Jun 1;169(6):2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Chen I. M., Kubo R., Tung K. S. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989 Aug 18;245(4919):749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- Talor E., Rose N. R. The induction of antigen-specific thymic regulatory cells in the mouse. Cell Immunol. 1988 Oct 1;116(1):24–34. doi: 10.1016/0008-8749(88)90206-7. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeira M., Gallily R. Interaction between thymocytes and thymus-derived macrophages. II. Engulfment of thymocytes by macrophages. Cell Immunol. 1988 Dec;117(2):277–288. doi: 10.1016/0008-8749(88)90118-9. [DOI] [PubMed] [Google Scholar]
- Zeira M., Gallily R., Stein I., Giloh H. Thymocyte maturation following interaction with thymus-derived macrophages. Cell Immunol. 1991 May;134(2):370–377. doi: 10.1016/0008-8749(91)90310-8. [DOI] [PubMed] [Google Scholar]
- Zöller M. Comparison between thymus- and spleen-derived B-cell hybridomas. Scand J Immunol. 1989 Aug;30(2):271–275. doi: 10.1111/j.1365-3083.1989.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Zöller M. Intrathymic T cell repertoire after prenatal trinitrobenzene-sulfonic acid-treatment. Cell Immunol. 1990 Mar;126(1):31–46. doi: 10.1016/0008-8749(90)90298-6. [DOI] [PubMed] [Google Scholar]
- Zöller M. Recognition of idiotypic structures in the thymus. Scand J Immunol. 1989 May;29(5):579–588. doi: 10.1111/j.1365-3083.1989.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Zöller M. Tolerization during pregnancy: impact on the development of antigen-specific help and suppression. Eur J Immunol. 1988 Dec;18(12):1937–1943. doi: 10.1002/eji.1830181211. [DOI] [PubMed] [Google Scholar]
- van Ewijk W., Ron Y., Monaco J., Kappler J., Marrack P., Le Meur M., Gerlinger P., Durand B., Benoist C., Mathis D. Compartmentalization of MHC class II gene expression in transgenic mice. Cell. 1988 May 6;53(3):357–370. doi: 10.1016/0092-8674(88)90156-0. [DOI] [PubMed] [Google Scholar]


