Abstract
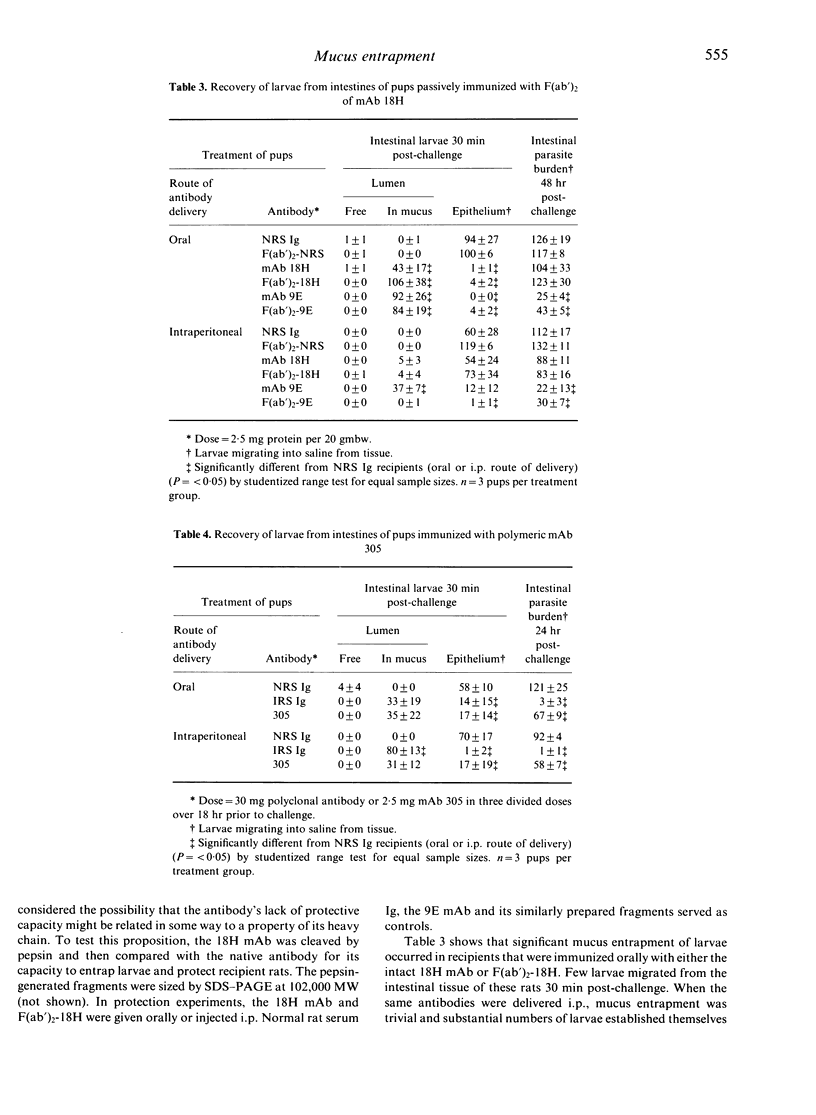
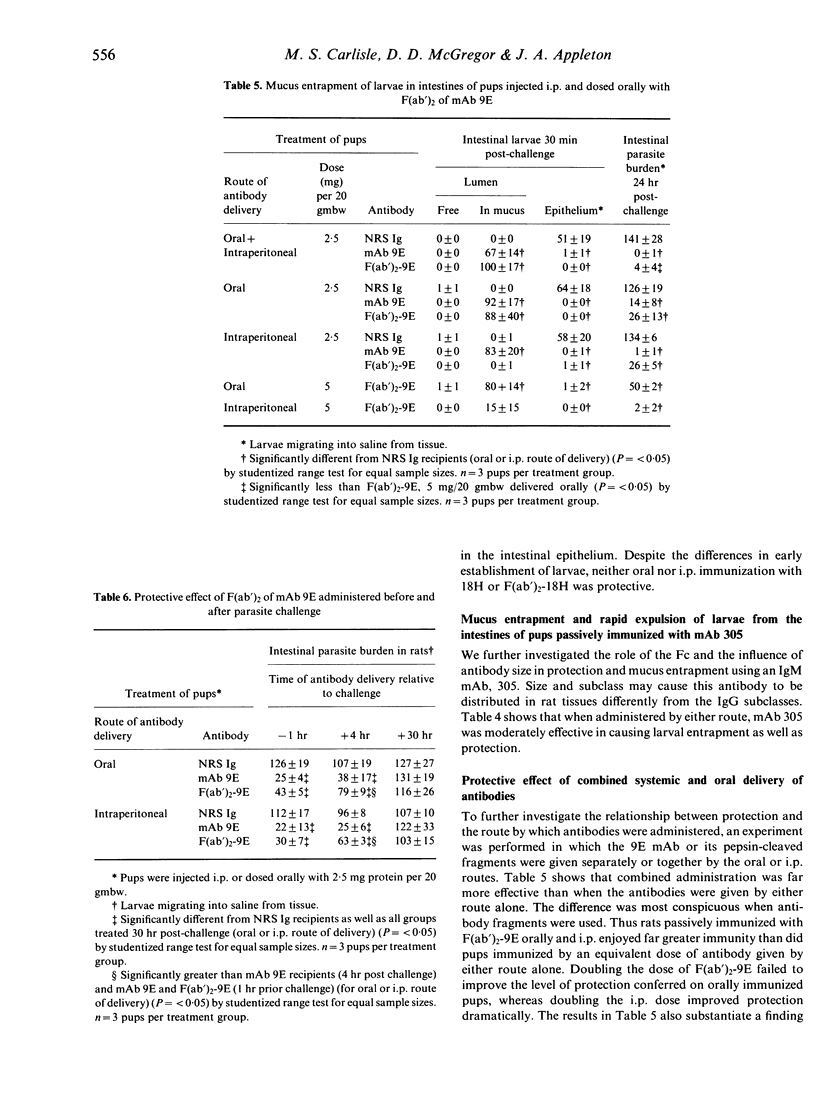
When an IgG2c monoclonal antibody specific for Trichinella spiralis muscle stage larvae was cleaved with pepsin to yield F(ab')2 fragments, the latter retained their capacity to cause mucus entrapment and rapid expulsion of larvae from the intestines of suckling rats. When fed to pups, the F(ab')2 fragments of this antibody and the F(ab')2 fragments of a similarly prepared IgG2a antibody caused mucus entrapment of muscle larvae (ML), demonstrating that trapping is not dependent upon the Fc region of the antibody molecule. Despite the fact that these two antibodies had similar specificities and that their F(ab')2 fragments caused larval entrapment in mucus, F(ab')2 fragments of the IgG2a antibody failed to protect rat pups. Fragments of the IgG2c antibody caused rapid expulsion when injected into pups, but the distribution of larvae was dramatically different from when the fragments were delivered orally. These results indicate that entrapment of T. spiralis in mucus is not in itself the cause of the expulsion. The more likely possibility is that antibody impedes a function of Trichinella spiralis that is related to the capacity of the parasite to reside in its epithelial niche.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton J. A., McGregor D. D. Characterization of the immune mediator of rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1987 Nov;62(3):477–484. [PMC free article] [PubMed] [Google Scholar]
- Appleton J. A., McGregor D. D. Life-phase specific induction and expression of rapid expulsion in rats suckling Trichinella spiralis-infected dams. Immunology. 1985 Jun;55(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Appleton J. A., McGregor D. D. Rapid expulsion of Trichinella spiralis in suckling rats. Science. 1984 Oct 5;226(4670):70–72. doi: 10.1126/science.6474191. [DOI] [PubMed] [Google Scholar]
- Appleton J. A., Schain L. R., McGregor D. D. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988 Nov;65(3):487–492. [PMC free article] [PubMed] [Google Scholar]
- Bell R. G., Adams L. S., Ogden R. W. Intestinal mucus trapping in the rapid expulsion of Trichinella spiralis by rats: induction and expression analyzed by quantitative worm recovery. Infect Immun. 1984 Jul;45(1):267–272. doi: 10.1128/iai.45.1.267-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthistle B. K., Kubo R. T., Brown W. R., Grey H. M. Studies on receptors for IgG on epithelial cells of the rat intestine. J Immunol. 1977 Aug;119(2):471–476. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlisle M. S., McGregor D. D., Appleton J. A. The role of mucus in antibody-mediated rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1990 May;70(1):126–132. [PMC free article] [PubMed] [Google Scholar]
- Douglass T. G., Speer C. A. Effects of intestinal contents from normal and immunized mice on sporozoites of Eimeria falciformis. J Protozool. 1985 Feb;32(1):156–163. doi: 10.1111/j.1550-7408.1985.tb03031.x. [DOI] [PubMed] [Google Scholar]
- McSweegan E., Burr D. H., Walker R. I. Intestinal mucus gel and secretory antibody are barriers to Campylobacter jejuni adherence to INT 407 cells. Infect Immun. 1987 Jun;55(6):1431–1435. doi: 10.1128/iai.55.6.1431-1435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F. Protection against nematodes by intestinal mucus. Adv Exp Med Biol. 1982;144:243–245. doi: 10.1007/978-1-4615-9254-9_37. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Wallace G. R. Immune exclusion and mucus trapping during the rapid expulsion of Nippostrongylus brasiliensis from primed rats. Immunology. 1981 Oct;44(2):419–429. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Jackson F., Newlands G., Appleyard W. T. Immune exclusion, a mechanism of protection against the ovine nematode Haemonchus contortus. Res Vet Sci. 1983 Nov;35(3):357–363. [PubMed] [Google Scholar]
- Rousseaux J., Rousseaux-Prévost R., Bazin H. Optimal conditions for the preparation of Fab and F(ab')2 fragments from monoclonal IgG of different rat IgG subclasses. J Immunol Methods. 1983 Nov 11;64(1-2):141–146. doi: 10.1016/0022-1759(83)90392-7. [DOI] [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]


