Abstract
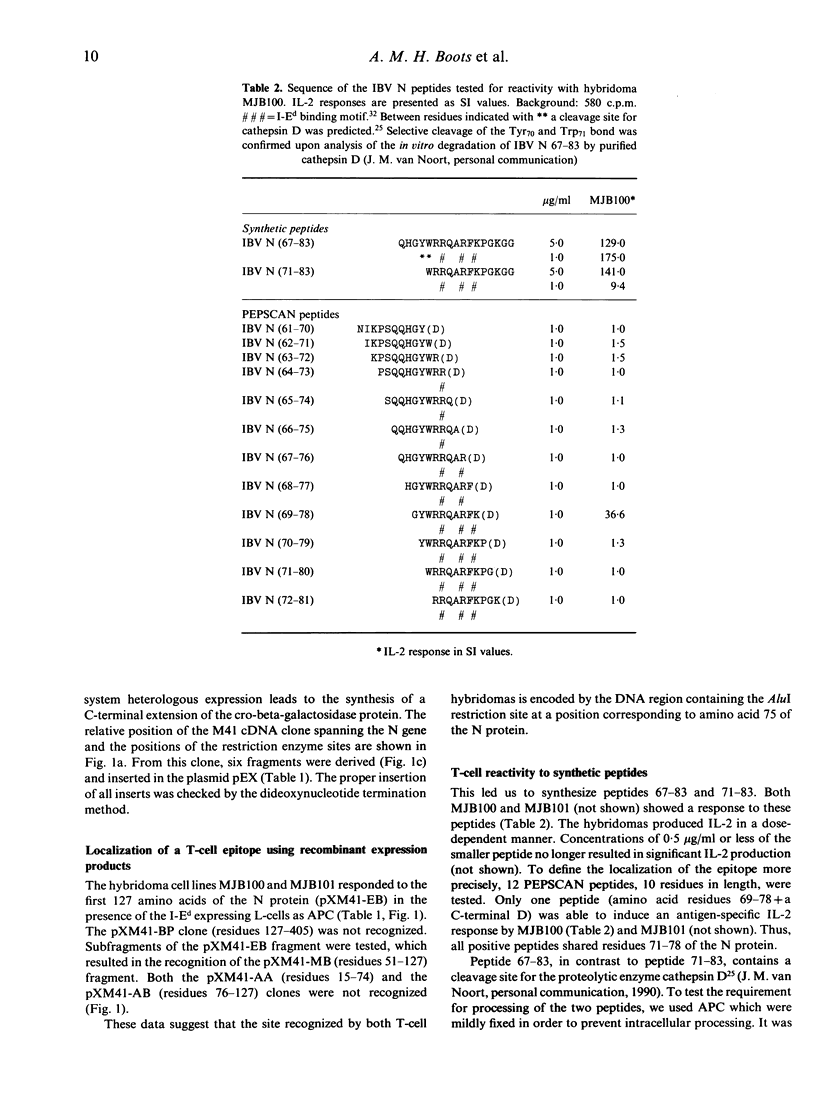
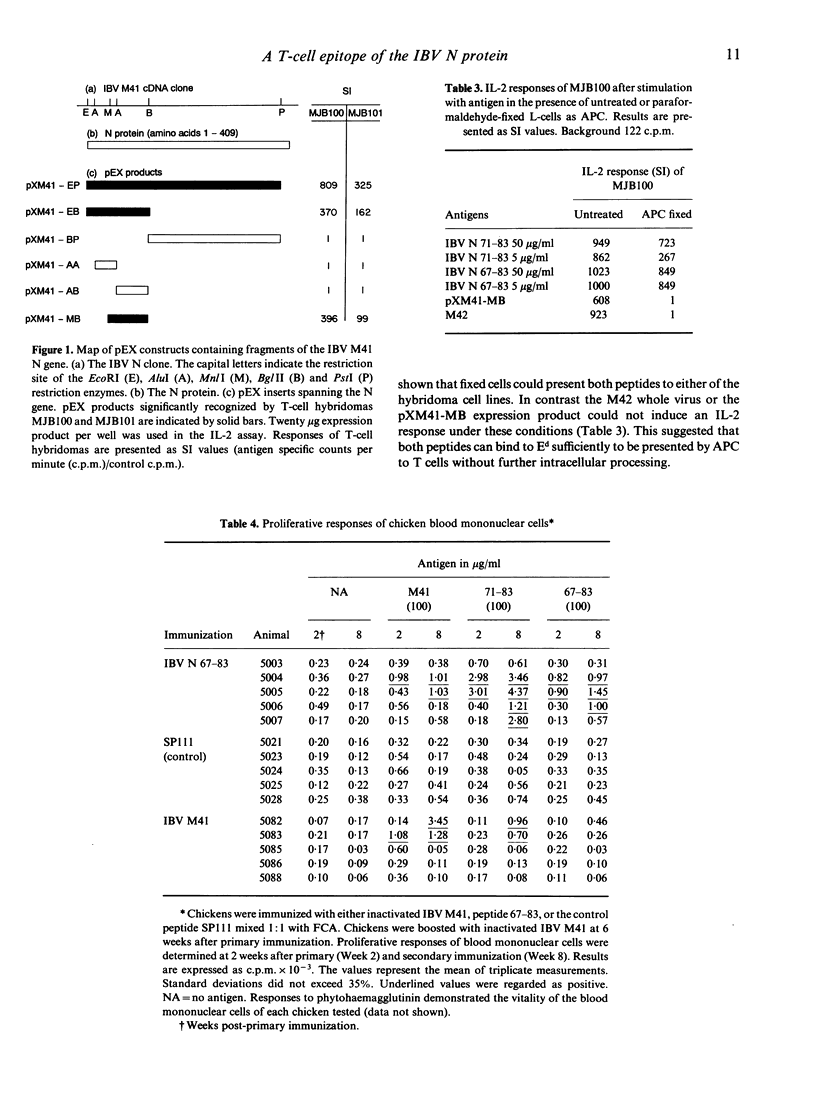
In a previous study, two murine T-cell hybridomas generated after immunization with infectious bronchitis virus (IBV) were shown to be responsive to the internally localized viral nucleocapsid protein. In the present study, the antigenic determinants were mapped using recombinant expression products and synthetic peptides. Both hybridomas recognized the region spanning amino acid residues 71 to 78 of the nucleocapsid protein. The experimentally determined epitope corresponded with predicted motifs. Both an I-Ed binding motif and a predicted cleavage site for the aspartyl protease cathepsin D were contained within the sequence. The epitope was shown to prime cellular immune responses to IBV in the chicken.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boots A. M., Kusters J. G., van der Zeijst B. A., Hensen E. J. The nucleocapsid protein of IBV comprises immunodominant determinants recognized by T-cells. Adv Exp Med Biol. 1990;276:189–197. doi: 10.1007/978-1-4684-5823-7_26. [DOI] [PubMed] [Google Scholar]
- Boots A. M., Van Lierop M. J., Kusters J. G., Van Kooten P. J., Van der Zeijst B. A., Hensen E. J. MHC class II-restricted T-cell hybridomas recognizing the nucleocapsid protein of avian coronavirus IBV. Immunology. 1991 Jan;72(1):10–14. [PMC free article] [PubMed] [Google Scholar]
- Boursnell M. E., Binns M. M., Foulds I. J., Brown T. D. Sequences of the nucleocapsid genes from two strains of avian infectious bronchitis virus. J Gen Virol. 1985 Mar;66(Pt 3):573–580. doi: 10.1099/0022-1317-66-3-573. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Ouyang C. S., Berzofsky J. A. Fine specificity of T cell recognition of the same peptide in association with different I-A molecules. J Immunol. 1989 Aug 1;143(3):771–779. [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus IBV glycopolypeptides: size of their polypeptide moieties and nature of their oligosaccharides. J Gen Virol. 1983 May;64(Pt 5):1187–1191. doi: 10.1099/0022-1317-64-5-1187. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus IBV: further evidence that the surface projections are associated with two glycopolypeptides. J Gen Virol. 1983 Aug;64(Pt 8):1787–1791. doi: 10.1099/0022-1317-64-8-1787. [DOI] [PubMed] [Google Scholar]
- Cease K. B., Margalit H., Cornette J. L., Putney S. D., Robey W. G., Ouyang C., Streicher H. Z., Fischinger P. J., Gallo R. C., DeLisi C. Helper T-cell antigenic site identification in the acquired immunodeficiency syndrome virus gp120 envelope protein and induction of immunity in mice to the native protein using a 16-residue synthetic peptide. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4249–4253. doi: 10.1073/pnas.84.12.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmann B., Gomard E., Kiény M. P., Guy B., Dreyfus F., Saimot A. G., Sereni D., Lévy J. P. An antigenic peptide of the HIV-1 NEF protein recognized by cytotoxic T lymphocytes of seropositive individuals in association with different HLA-B molecules. Eur J Immunol. 1989 Dec;19(12):2383–2386. doi: 10.1002/eji.1830191231. [DOI] [PubMed] [Google Scholar]
- Curling E., Slade C., Hutchinson I., Morris P. Isolation of rat/rat T cell hybrids using W/Fu C58(NT)D as fusion partner. J Immunol Methods. 1988 Apr 6;108(1-2):171–178. doi: 10.1016/0022-1759(88)90416-4. [DOI] [PubMed] [Google Scholar]
- Davelaar F. G., Kouwenhoven B., Burger A. G. Occurrence and significance of infectious bronchitis virus variant strains in egg and broiler production in the Netherlands. Vet Q. 1984 Jul;6(3):114–120. doi: 10.1080/01652176.1984.9693924. [DOI] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Appella E., Sette A. Characterization of a naturally processed MHC class II-restricted T-cell determinant of hen egg lysozyme. Nature. 1989 Dec 7;342(6250):682–684. doi: 10.1038/342682a0. [DOI] [PubMed] [Google Scholar]
- Gao X. M., Liew F. Y., Tite J. P. A dominant Th epitope in influenza nucleoprotein. Analysis of the fine specificity and functional repertoire of T cells recognizing a single determinant. J Immunol. 1990 Apr 1;144(7):2730–2737. [PubMed] [Google Scholar]
- Germain R. N., Quill H. Unexpected expression of a unique mixed-isotype class II MHC molecule by transfected L-cells. Nature. 1986 Mar 6;320(6057):72–75. doi: 10.1038/320072a0. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Jordi B. J., Kremers D. A., Kusters H. G., van der Zeijst B. A. Nucleotide sequence of the gene coding for the peplomer protein (= spike protein) of infectious bronchitis virus, strain D274. Nucleic Acids Res. 1989 Aug 25;17(16):6726–6726. doi: 10.1093/nar/17.16.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Kovac Z., Schwartz R. H. The molecular basis of the requirement for antigen processing of pigeon cytochrome c prior to T cell activation. J Immunol. 1985 May;134(5):3233–3240. [PubMed] [Google Scholar]
- Kusters J. G., Jager E. J., Lenstra J. A., Koch G., Posthumus W. P., Meloen R. H., van der Zeijst B. A. Analysis of an immunodominant region of infectious bronchitis virus. J Immunol. 1989 Oct 15;143(8):2692–2698. [PubMed] [Google Scholar]
- Kusters J. G., Niesters H. G., Bleumink-Pluym N. M., Davelaar F. G., Horzinek M. C., Van der Zeijst B. A. Molecular epidemiology of infectious bronchitis virus in The Netherlands. J Gen Virol. 1987 Feb;68(Pt 2):343–352. doi: 10.1099/0022-1317-68-2-343. [DOI] [PubMed] [Google Scholar]
- Kusters J. G., Niesters H. G., Lenstra J. A., Horzinek M. C., van der Zeijst B. A. Phylogeny of antigenic variants of avian coronavirus IBV. Virology. 1989 Mar;169(1):217–221. doi: 10.1016/0042-6822(89)90058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra J. A., Kusters J. G., Koch G., van der Zeijst B. A. Antigenicity of the peplomer protein of infectious bronchitis virus. Mol Immunol. 1989 Jan;26(1):7–15. doi: 10.1016/0161-5890(89)90014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Niesters H. G., Lenstra J. A., Spaan W. J., Zijderveld A. J., Bleumink-Pluym N. M., Hong F., van Scharrenburg G. J., Horzinek M. C., van der Zeijst B. A. The peplomer protein sequence of the M41 strain of coronavirus IBV and its comparison with Beaudette strains. Virus Res. 1986 Aug;5(2-3):253–263. doi: 10.1016/0168-1702(86)90022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G. F., van der Heijden R. W., Tijhaar E., Poelen M. C., Carlson J., Osterhaus A. D., UytdeHaag F. G. Establishment and characterization of canine parvovirus-specific murine CD4+ T cell clones and their use for the delineation of T cell epitopes. J Gen Virol. 1990 May;71(Pt 5):1095–1102. doi: 10.1099/0022-1317-71-5-1095. [DOI] [PubMed] [Google Scholar]
- Sette A., Adorini L., Appella E., Colón S. M., Miles C., Tanaka S., Ehrhardt C., Doria G., Nagy Z. A., Buus S. Structural requirements for the interaction between peptide antigens and I-Ed molecules. J Immunol. 1989 Nov 15;143(10):3289–3294. [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Sefton B. M. Coronavirus multiplication: locations of genes for virion proteins on the avian infectious bronchitis virus genome. J Virol. 1984 Apr;50(1):22–29. doi: 10.1128/jvi.50.1.22-29.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms L. M., Bracewell C. D., Alexander D. J. Cell mediated and humoral immune response in chickens infected with avian infectious bronchitis. Br Vet J. 1980 Jul-Aug;136(4):349–346. doi: 10.1016/s0007-1935(17)32237-6. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Van der Zee R., Van Eden W., Meloen R. H., Noordzij A., Van Embden J. D. Efficient mapping and characterization of a T cell epitope by the simultaneous synthesis of multiple peptides. Eur J Immunol. 1989 Jan;19(1):43–47. doi: 10.1002/eji.1830190108. [DOI] [PubMed] [Google Scholar]
- van Noort J. M., van der Drift A. C. The selectivity of cathepsin D suggests an involvement of the enzyme in the generation of T-cell epitopes. J Biol Chem. 1989 Aug 25;264(24):14159–14164. [PubMed] [Google Scholar]


