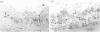Abstract
Experimental autoimmune uveoretinitis (EAU) is a well-characterized model of immune-mediated intraocular inflammation. The intraocular infiltrate in EAU consists predominantly of T lymphocytes. The in vivo production of interferon-gamma (IFN-gamma) by these T cells was investigated immunohistochemically and by in situ hybridization using a cDNA probe to rat IFN-gamma mRNA. Positive localization of IFN-gamma mRNA began simultaneously with disease onset and increased as the inflammatory tissue destruction progressed. The positive signal was seen on cells in the retina, uveal tract and extraocular region where collections of inflammatory cells contained many T lymphocytes. Numerous cells in these locations also stained positively immunohistochemically for IFN-gamma. These results indicate that the in vivo production of IFN-gamma within the eye could play a role in the immune regulation of intraocular inflammatory disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chan C. C., Detrick B., Nussenblatt R. B., Palestine A. G., Fujikawa L. S., Hooks J. J. HLA-DR antigens on retinal pigment epithelial cells from patients with uveitis. Arch Ophthalmol. 1986 May;104(5):725–729. doi: 10.1001/archopht.1986.01050170115034. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Hooks J. J., Nussenblatt R. B., Detrick B. Expression of Ia antigen on retinal pigment epithelium in experimental autoimmune uveoretinitis. Curr Eye Res. 1986 Apr;5(4):325–330. doi: 10.3109/02713688609020059. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Mochizuki M., Nussenblatt R. B., Palestine A. G., McAllister C., Gery I., BenEzra D. T-lymphocyte subsets in experimental autoimmune uveitis. Clin Immunol Immunopathol. 1985 Apr;35(1):103–110. doi: 10.1016/0090-1229(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Chen L. K., Tourvieille B., Burns G. F., Bach F. H., Mathieu-Mahul D., Sasportes M., Bensussan A. Interferon: a cytotoxic T lymphocyte differentiation signal. Eur J Immunol. 1986 Jul;16(7):767–770. doi: 10.1002/eji.1830160709. [DOI] [PubMed] [Google Scholar]
- Dijkema R., van der Meide P. H., Pouwels P. H., Caspers M., Dubbeld M., Schellekens H. Cloning and expression of the chromosomal immune interferon gene of the rat. EMBO J. 1985 Mar;4(3):761–767. doi: 10.1002/j.1460-2075.1985.tb03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey C., Cozette J., Faure J. P. A simple and rapid method for isolation of retinal S antigen. Ophthalmic Res. 1982;14(4):249–255. doi: 10.1159/000265199. [DOI] [PubMed] [Google Scholar]
- Hamel C. P., Detrick B., Hooks J. J. Evaluation of Ia expression in rat ocular tissues following inoculation with interferon-gamma. Exp Eye Res. 1990 Feb;50(2):173–182. doi: 10.1016/0014-4835(90)90228-m. [DOI] [PubMed] [Google Scholar]
- Hooks J. J., Chan C. C., Detrick B. Identification of the lymphokines, interferon-gamma and interleukin-2, in inflammatory eye diseases. Invest Ophthalmol Vis Sci. 1988 Sep;29(9):1444–1451. [PubMed] [Google Scholar]
- Liversidge J. M., Sewell H. F., Forrester J. V. Human retinal pigment epithelial cells differentially express MHC class II (HLA, DP, DR and DQ) antigens in response to in vitro stimulation with lymphokine or purified IFN-gamma. Clin Exp Immunol. 1988 Sep;73(3):489–494. [PMC free article] [PubMed] [Google Scholar]
- Liversidge J., Sewell H. F., Thomson A. W., Forrester J. V. Lymphokine-induced MHC class II antigen expression on cultured retinal pigment epithelial cells and the influence of cyclosporin A. Immunology. 1988 Feb;63(2):313–317. [PMC free article] [PubMed] [Google Scholar]
- Mason D., Powrie F. Memory CD4+ T cells in man form two distinct subpopulations, defined by their expression of isoforms of the leucocyte common antigen, CD45. Immunology. 1990 Aug;70(4):427–433. [PMC free article] [PubMed] [Google Scholar]
- McKnight A. J., Barclay A. N., Mason D. W. Molecular cloning of rat interleukin 4 cDNA and analysis of the cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991 May;21(5):1187–1194. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. The MRC OX-22- CD4+ T cells that help B cells in secondary immune responses derive from naive precursors with the MRC OX-22+ CD4+ phenotype. J Exp Med. 1989 Mar 1;169(3):653–662. doi: 10.1084/jem.169.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Bacon P. A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from human peripheral blood and by human CD4+ T cell clones. J Immunol. 1989 Aug 1;143(3):907–912. [PubMed] [Google Scholar]
- Simon M. M., Landolfo S., Diamantstein T., Hochgeschwender U. Antigen- and lectin-sensitized murine cytolytic T lymphocyte-precursors require both interleukin 2 and endogenously produced immune (gamma) interferon for their growth and differentiation into effector cells. Curr Top Microbiol Immunol. 1986;126:173–185. doi: 10.1007/978-3-642-71152-7_21. [DOI] [PubMed] [Google Scholar]