Abstract
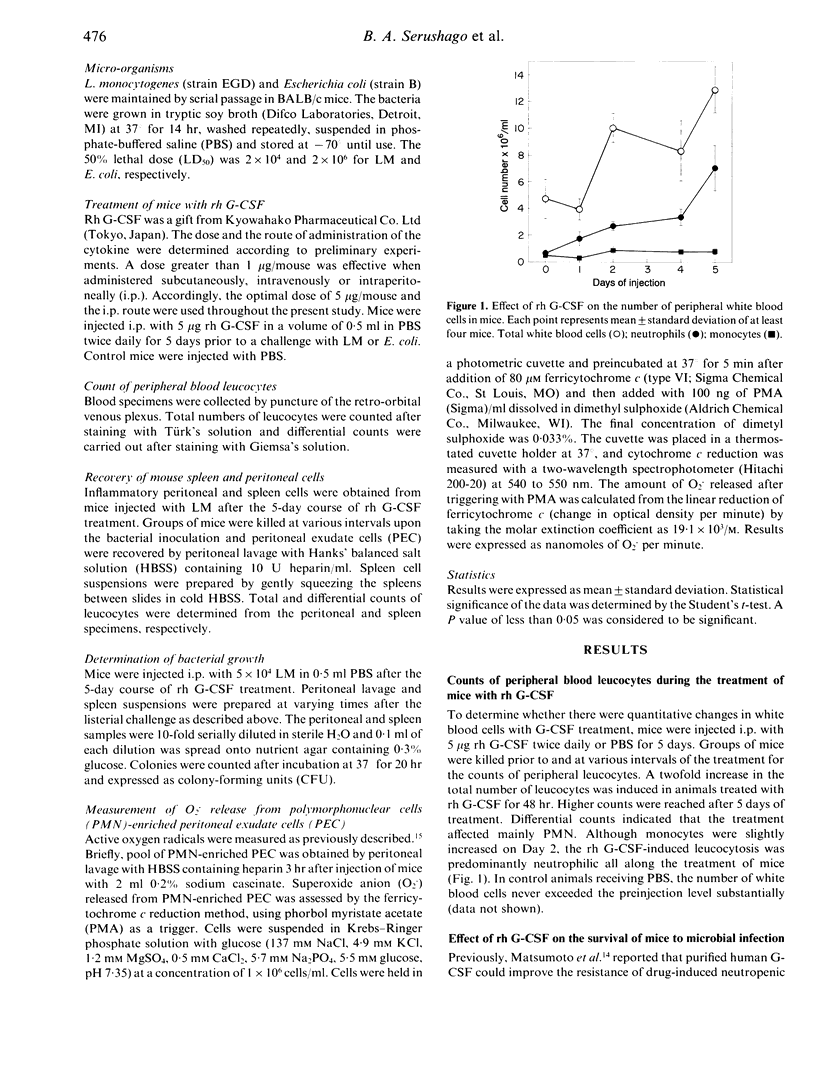
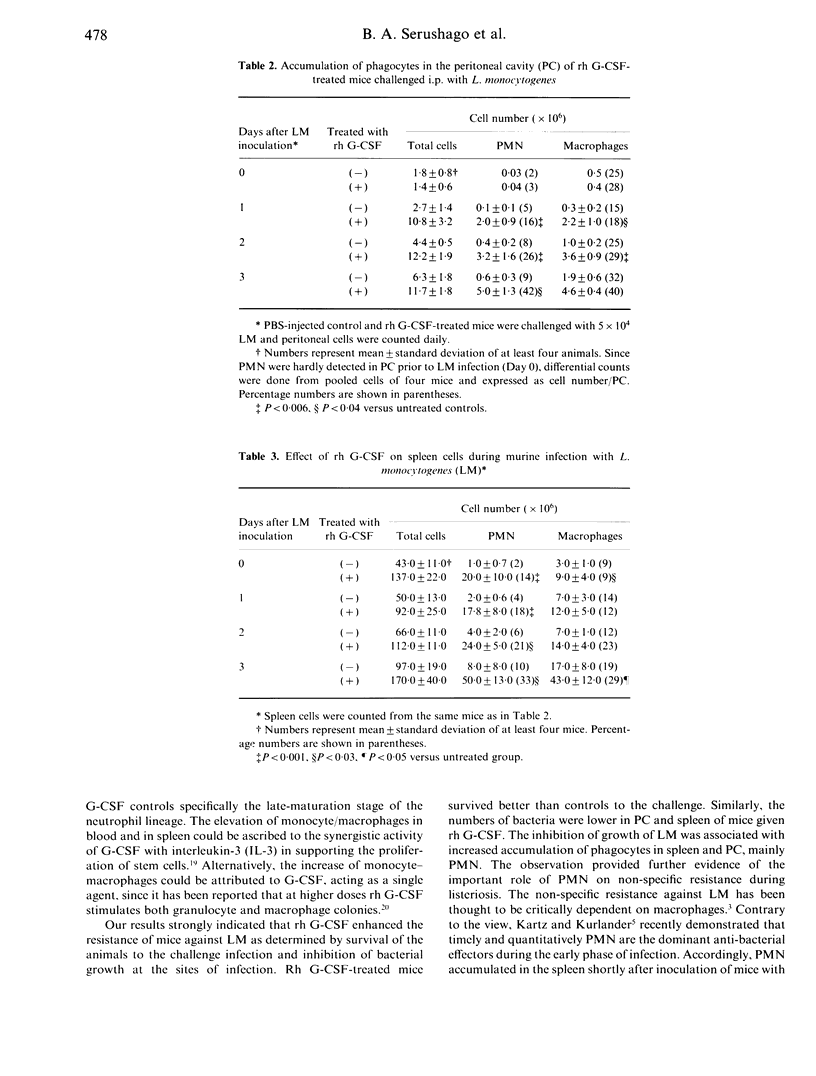
Recombinant human granulocyte colony-stimulating factor (rh G-CSF) enhanced resistance of mice against Listeria monocytogenes (LM) as determined by survival and bacterial growth. Mice pretreated with rh G-CSF twice daily for 5 days survived better than untreated animals to the challenge with LM. Number of bacteria in peritoneal cavity (PC) and spleen was lower in treated mice than that in the control group. Rh G-CSF increased mainly polymorphonuclear cells (PMN) in blood and spleen. After LM inoculation, a larger number of PMN and monocyte-macrophages accumulated in PC and spleen of tested mice. In addition, PMN primed in vivo with rh G-CSF released more superoxide anions when stimulated with phorbol myristate acetate. The inhibition of bacterial growth in PC and spleen could be ascribed to the accumulation of phagocytic cells at the infection sites and the increased oxidative metabolism. The results provided further evidence of the important contribution of G-CSF and neutrophils, as target cells, to the host defence against the intracellular bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairo M. S., Mauss D., Kommareddy S., Norris K., van de Ven C., Modanlou H. Prophylactic or simultaneous administration of recombinant human granulocyte colony stimulating factor in the treatment of group B streptococcal sepsis in neonatal rats. Pediatr Res. 1990 Jun;27(6):612–616. doi: 10.1203/00006450-199006000-00016. [DOI] [PubMed] [Google Scholar]
- Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988 Jan;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. M., Hines D. K., Korach E. S., Ratzkin B. J. In vivo activation of neutrophil function in hamsters by recombinant human granulocyte colony-stimulating factor. Infect Immun. 1988 Nov;56(11):2861–2865. doi: 10.1128/iai.56.11.2861-2865.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D. Colony-stimulating factors in vivo and in vitro. Immunol Today. 1988 Apr;9(4):97–98. doi: 10.1016/0167-5699(88)91274-1. [DOI] [PubMed] [Google Scholar]
- Gabrilove J. L., Jakubowski A. Granulocyte colony-stimulating factor: preclinical and clinical studies. Hematol Oncol Clin North Am. 1989 Sep;3(3):427–440. [PubMed] [Google Scholar]
- Ikebuchi K., Clark S. C., Ihle J. N., Souza L. M., Ogawa M. Granulocyte colony-stimulating factor enhances interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1988 May;85(10):3445–3449. doi: 10.1073/pnas.85.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongshavn P. A., Skamene E. The role of natural resistance in protection of the murine host from listeriosis. Clin Invest Med. 1984;7(4):253–257. [PubMed] [Google Scholar]
- Kratz S. S., Kurlander R. J. Characterization of the pattern of inflammatory cell influx and cytokine production during the murine host response to Listeria monocytogenes. J Immunol. 1988 Jul 15;141(2):598–606. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Virgin H. W., 4th, Unanue E. R. In vivo and in vitro expression of macrophage membrane interleukin 1 in response to soluble and particulate stimuli. J Immunol. 1986 Jul 1;137(1):10–14. [PubMed] [Google Scholar]
- Leizer T., Cebon J., Layton J. E., Hamilton J. A. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. Induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990 Nov 15;76(10):1989–1996. [PubMed] [Google Scholar]
- Miyata M., Mitsuyama M., Ogata N., Nomoto K. Protective mechanisms against infection by Listeria monocytogenes: accumulation and activation of macrophages. J Clin Lab Immunol. 1984 Mar;13(3):111–115. [PubMed] [Google Scholar]
- Morstyn G., Campbell L., Souza L. M., Alton N. K., Keech J., Green M., Sheridan W., Metcalf D., Fox R. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988 Mar 26;1(8587):667–672. doi: 10.1016/s0140-6736(88)91475-4. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer D. W., Donowitz G. R., Mandell G. L. Polymorphonuclear neutrophils: an effective antimicrobial force. Rev Infect Dis. 1989 Nov-Dec;11 (Suppl 7):S1532–S1544. doi: 10.1093/clinids/11.supplement_7.s1532. [DOI] [PubMed] [Google Scholar]
- Steinbeck M. J., Roth J. A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989 Jul-Aug;11(4):549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- Tatsukawa K., Mitsuyama M., Takeya K., Nomoto K. Differing contribution of polymorphonuclear cells and macrophages to protection of mice against Listeria monocytogenes and Pseudomonas aeruginosa. J Gen Microbiol. 1979 Nov;115(1):161–166. doi: 10.1099/00221287-115-1-161. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W. Physiology of granulocyte and macrophage colony-stimulating factors in host defense. Hematol Oncol Clin North Am. 1989 Sep;3(3):401–409. [PubMed] [Google Scholar]
- Wing E. J., Barczynski L. C., Waheed A., Shadduck R. K. Effect of Listeria monocytogenes infection on serum levels of colony-stimulating factor and number of progenitor cells in immune and nonimmune mice. Infect Immun. 1985 Aug;49(2):325–328. doi: 10.1128/iai.49.2.325-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Ohga S., Takeda Y., Nomoto K. Effects of stimulated or immunologically activated macrophages on the induction of immune responses to Listeria monocytogenes. Immunobiology. 1990 Feb;180(2-3):124–137. doi: 10.1016/S0171-2985(11)80323-2. [DOI] [PubMed] [Google Scholar]


