Abstract
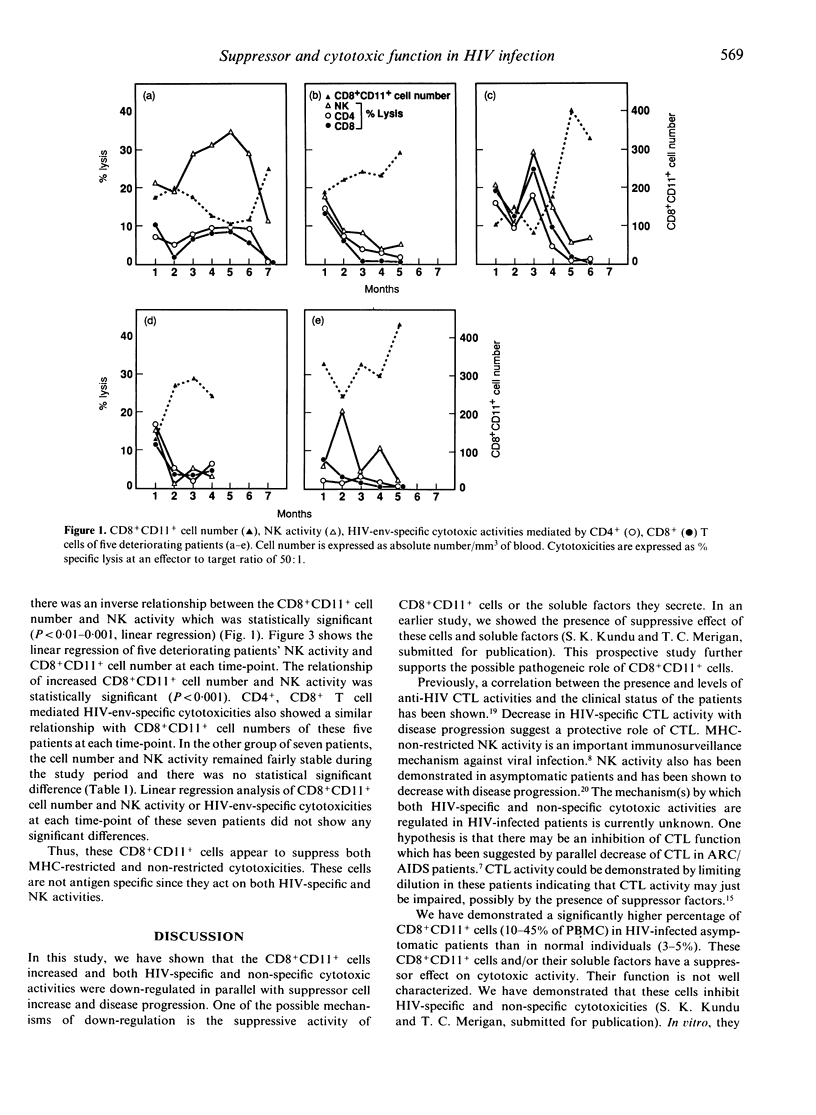
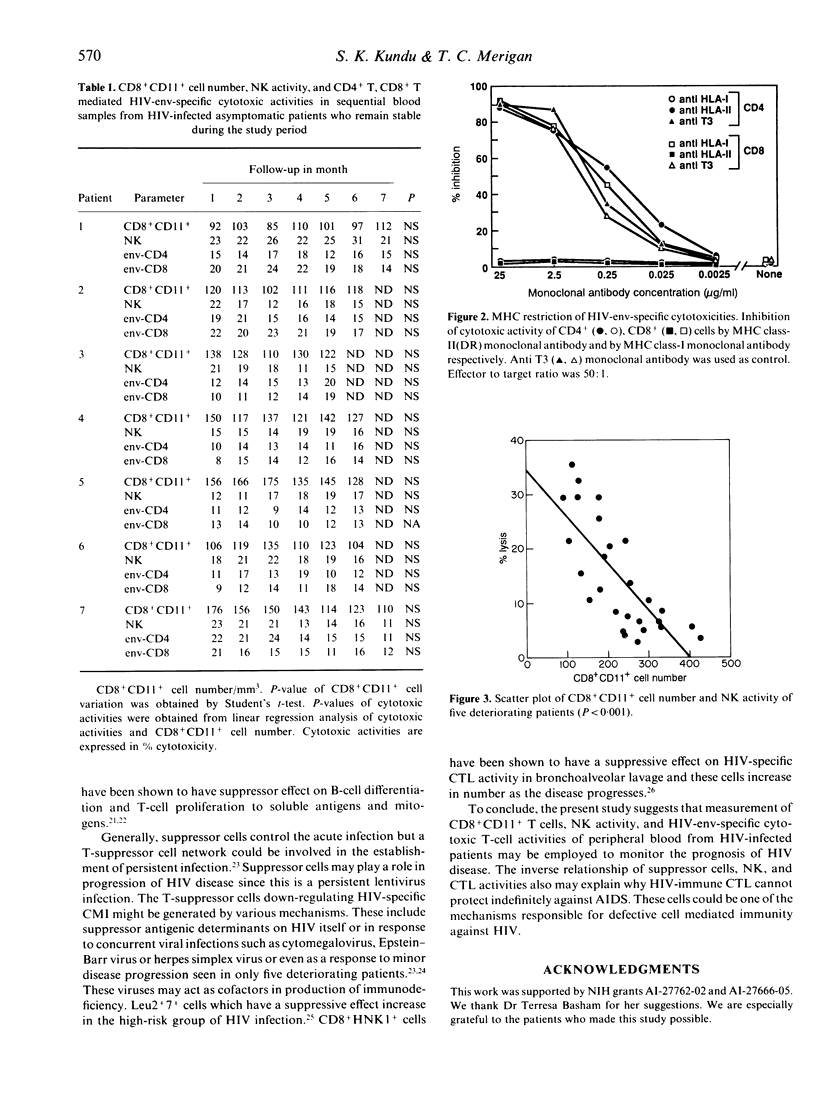
Profiles of CD8+CD11+ T suppressor cells, human immunodeficiency virus (HIV)-env-specific cytotoxic T-lymphocyte (CTL) activities, and natural killer (NK) cell activity were studied in 12 asymptomatic untreated HIV-infected patients. These patients were followed for 4-7 months. NK activity, HIV-env-specific CTL activities mediated by CD4+, CD8+ T cells and CD8+CD11+ T-suppressor cell number remained stable in seven patients during the study period. Alternatively, NK and HIV-specific CTL activities decreased and CD8+CD11+ cell number increased in five patients whose CD4+ T-cell number fell, and in four of these five patients serum p24 antigen level increased, and they developed minor clinical signs of disease progression during the study period. CD8+CD11+ cells are present in higher percentage (10-45% of peripheral blood mononuclear cells) in these HIV-infected patients as compared to those in normal individuals (3-5%). Our results suggest that CD8+CD11+ cells, NK, and HIV-specific cytotoxic activities may be helpful in monitoring prognosis of HIV infection. These observations also suggest that CD8+CD11+ cells may play an important role in the failure of host immune defences against HIV.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzofsky J. A., Bensussan A., Cease K. B., Bourge J. F., Cheynier R., Lurhuma Z., Salaün J. J., Gallo R. C., Shearer G. M., Zagury D. Antigenic peptides recognized by T lymphocytes from AIDS viral envelope-immune humans. Nature. 1988 Aug 25;334(6184):706–708. doi: 10.1038/334706a0. [DOI] [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Zajac R. A., Boswell R. N., Gebel H. M., Takahashi H., Berzofsky J. A., Shearer G. M. Detection of cytotoxic T lymphocytes specific for synthetic peptides of gp160 in HIV-seropositive individuals. J Immunol. 1991 Apr 1;146(7):2214–2219. [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Nishanian P. G., Schmid I., Hultin L. E., Cheng H. L., Detels R. Selective alterations in immunoregulatory lymphocyte subsets in early HIV (human T-lymphotropic virus type III/lymphadenopathy-associated virus) infection. J Clin Immunol. 1987 Mar;7(2):140–150. doi: 10.1007/BF00916008. [DOI] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Joly P., Guillon J. M., Mayaud C., Plata F., Theodorou I., Denis M., Debre P., Autran B. Cell-mediated suppression of HIV-specific cytotoxic T lymphocytes. J Immunol. 1989 Oct 1;143(7):2193–2201. [PubMed] [Google Scholar]
- Koenig S., Earl P., Powell D., Pantaleo G., Merli S., Moss B., Fauci A. S. Group-specific, major histocompatibility complex class I-restricted cytotoxic responses to human immunodeficiency virus 1 (HIV-1) envelope proteins by cloned peripheral blood T cells from an HIV-1-infected individual. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8638–8642. doi: 10.1073/pnas.85.22.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landay A., Gartland G. L., Clement L. T. Characterization of a phenotypically distinct subpopulation of Leu-2+ cells that suppresses T cell proliferative responses. J Immunol. 1983 Dec;131(6):2757–2761. [PubMed] [Google Scholar]
- Lewis D. E., Puck J. M., Babcock G. F., Rich R. R. Disproportionate expansion of a minor T cell subset in patients with lymphadenopathy syndrome and acquired immunodeficiency syndrome. J Infect Dis. 1985 Mar;151(3):555–559. doi: 10.1093/infdis/151.3.555. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren K., Böttiger B., Biberfeld G., Karlson A., Fenyö E. M., Jondal M. Antibody-dependent cellular cytotoxicity-inducing antibodies against human immunodeficiency virus. Presence at different clinical stages. J Immunol. 1987 Oct 1;139(7):2263–2267. [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Wong L., Fujimiya Y., Chang W. C., Horwitz D. A., Bloom B. R., Rea T. H., Pattengale P. K. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986 Nov 1;137(9):2831–2834. [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Tanneau-Salvadori F., Regnault A., Lopez O., Sansonetti P., Guy B., Kieny M. P., Fournel J. J., Montagnier L. Human immunodeficiency virus-specific cytotoxic responses of seropositive individuals: distinct types of effector cells mediate killing of targets expressing gag and env proteins. J Virol. 1989 May;63(5):2270–2277. doi: 10.1128/jvi.63.5.2270-2277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Näher H., Stroehmann I. Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-specific and HLA-restricted cytotoxic T-cell clones. Nature. 1988 Sep 8;335(6186):178–181. doi: 10.1038/335178a0. [DOI] [PubMed] [Google Scholar]
- Southern P., Oldstone M. B. Medical consequences of persistent viral infection. N Engl J Med. 1986 Feb 6;314(6):359–367. doi: 10.1056/NEJM198602063140606. [DOI] [PubMed] [Google Scholar]
- Tyler D. S., Stanley S. D., Nastala C. A., Austin A. A., Bartlett J. A., Stine K. C., Lyerly H. K., Bolognesi D. P., Weinhold K. J. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection. Humoral and cellular defects. J Immunol. 1990 May 1;144(9):3375–3384. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Ortaldo J. R., Herberman R. B., Reynolds C. W. Analysis of T cell receptors in highly purified rat and human large granular lymphocytes (LGL): lack of functional 1.3 kb beta-chain mRNA. J Immunol. 1986 Apr 1;136(7):2701–2704. [PubMed] [Google Scholar]


