Abstract
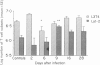
Infection in mice with an attenuated strain of Salmonella abortusovis (SAO), a specific pathogen for sheep, was used as a convenient model to understand further the induced immunity against SAO. The hypovirulent Rv6 strain, subcutaneously inoculated in salmonella-susceptible BALB/cby (Itys) mice, colonized the spleen and the liver in less than 6 days post-infection (PI) to be cleared after Day 28 PI. Simultaneously, an increase in spleen cell numbers, splenomegaly and hepatic granulomatous lesions developed to a maximum level on Day 9 PI. In spleen of uninfected mice, the number of Thy-1.2+ cells represents twice the number of surface immunoglobulin-positive cells (sIg+). Cytofluorometric analysis of the spleen lymphoid cell subsets showed a significant increase (10 times, P less than 0.05) in the number of sIg+ cells from Day 6 to Day 28 PI compared to control values. The number of Thy-1.2+ cells also significantly increased, to a lesser degree than the sIg+ cells, on Day 2 and on Day 16 PI (twice control values, P less than 0.05), but decreased on Day 6 PI compared to Day 2 PI. The highest L3T4+:Lyt-2+ ratio was observed on Day 2 PI and the lowest on Day 9 PI. On Day 28 PI, the number of sIg+ cells was still greater than the number of Thy-1.2+ cells. The granulomatous lesions were observed in the liver as early as Day 2 PI and their frequency was maximal on Day 9 PI. Immunohistochemical analysis of the granulomatous lesions showed that macrophages (F4/80+, Mac1+) were the basic cells and that L3T4+ cells were the predominant T cells. In well-developed granulomas observed on Day 9 PI, macrophages were in the centre whereas L3T4+ T cells were preferentially located at the periphery. T cells expressing Lyt-2 antigen were rarely detected. Variations in the proportion of lymphoid cells in the spleen and in hepatic granulomatous lesions suggest different and complementary effector mechanisms in induced immunity against SAO.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Lehmeyer J., Forman C. Phagocytosis and killing of salmonella typhimurium by peritoneal exudate cells. Infect Immun. 1981 Aug;33(2):380–388. doi: 10.1128/iai.33.2.380-388.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Flesch I., Kaufmann S. H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988 Jan;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- Denis M., Forget A., Miailhe A. C., Pelletier M., Skamene E. Evolution of cell types and T-cell subsets in the spleens of Mycobacterium bovis BCG-resistant and M. bovis BCG-susceptible strains of mice after infection with M. bovis BCG. Infect Immun. 1985 Jul;49(1):253–255. doi: 10.1128/iai.49.1.253-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Guilloteau L., Pépin M., Pardon P., Le Pape A. Recruitment of 99m-technetium- or 111-indium-labelled polymorphonuclear leucocytes in experimentally induced pyogranulomas in lambs. J Leukoc Biol. 1990 Oct;48(4):343–352. doi: 10.1002/jlb.48.4.343. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantier F., Pardon P., Marly J. Immunogenicity of a low-virulence vaccinal strain against Salmonella abortus-ovis infection in mice. Infect Immun. 1983 May;40(2):601–607. doi: 10.1128/iai.40.2.601-607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantier F., Pardon P., Marly J. Vaccinal properties of Salmonella abortus ovis mutants for streptomycin: screening with a murine model. Infect Immun. 1981 Nov;34(2):492–497. doi: 10.1128/iai.34.2.492-497.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Wang X. M., Hsu H. S., Mumaw V. R., Nakoneczna I. Electron microscopic studies on the location of bacterial proliferation in the liver in murine salmonellosis. Br J Exp Pathol. 1987 Aug;68(4):539–550. [PMC free article] [PubMed] [Google Scholar]
- Marecki N. M., Hsu H. S., Mayo D. R. Cellular and humoral aspects of host resistance in murine salmonellosis. Br J Exp Pathol. 1975 Jun;56(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- McGrath J. M., Stewart G. J. The effects of endotoxin on vascular endothelium. J Exp Med. 1969 May 1;129(5):833–848. doi: 10.1084/jem.129.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Niedobitek G., Stein H., Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989 Aug 1;170(2):589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Gebhard J. F., Taylor C. R., Rea T. H. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin Exp Immunol. 1983 Jul;53(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Nakoneczna I., Hsu H. S. Histopathological study of protective immunity against murine salmonellosis induced by killed vaccine. Infect Immun. 1983 Jan;39(1):423–430. doi: 10.1128/iai.39.1.423-430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Vilde F., Ronco E. Host response to infection with a temperature-sensitive mutant of Salmonella typhimurium in a susceptible and a resistant strain of mice. Infect Immun. 1985 Sep;49(3):523–527. doi: 10.1128/iai.49.3.523-527.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbering P. H., van der Heide G. A., van Furth R. Immunocytochemical analysis of cellular responses to BCG. Clin Exp Immunol. 1989 Jan;75(1):147–154. [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Takacs L., Hahn H. Dynamics of T cells of L3T4 and Ly 2 phenotype within granulomas in murine listeriosis. Clin Exp Immunol. 1985 Jun;60(3):559–564. [PMC free article] [PubMed] [Google Scholar]
- Ohga S., Yoshikai Y., Takeda Y., Hiromatsu K., Nomoto K. Sequential appearance of gamma/delta- and alpha/beta-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur J Immunol. 1990 Mar;20(3):533–538. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- Pardon P., Marly J. Experimental Salmonella abortus ovis infection of normal or primo-infected CD1 mice. Ann Microbiol (Paris) 1979 Jul;130B(1):21–28. [PubMed] [Google Scholar]
- Pardon P., Sanchis R., Marly J., Lantier F., Guilloteau L., Buzoni-Gatel D., Oswald I. P., Pépin M., Kaeffer B., Berthon P. Experimental ovine salmonellosis (Salmonella abortusovis): pathogenesis and vaccination. Res Microbiol. 1990 Sep-Oct;141(7-8):945–953. doi: 10.1016/0923-2508(90)90134-c. [DOI] [PubMed] [Google Scholar]
- Pardon P., Sanchis R., Marly J., Lantier F., Pépin M., Popoff M. Salmonellose ovine due à Salmonella abortusovis. Ann Rech Vet. 1988;19(4):221–235. [PubMed] [Google Scholar]
- Pelletier M., Forget A., Bourassa D., Gros P., Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982 Nov;129(5):2179–2185. [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Brzezicki M. J., Griggs N., Mahrer S. The role of natural killer cells in experimental murine salmonellosis. Nat Immun Cell Growth Regul. 1989;8(6):331–337. [PubMed] [Google Scholar]
- Stabel T. J., Mayfield J. E., Tabatabai L. B., Wannemuehler M. J. Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect Immun. 1990 Jul;58(7):2048–2055. doi: 10.1128/iai.58.7.2048-2055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]