Abstract
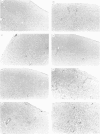
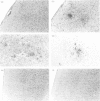
Studies were performed in a murine model to determine if there is genetic control of the development of toxoplasmic encephalitis. Ten weeks after infection with the ME49 strain of Toxoplasma gondii, mice with the H-2b haplotype (C57BL/6, C57BL/10) and H-2k haplotype (C3H/He, CBA/J) developed remarkable inflammatory changes in their brains, whereas mice with the H-2a haplotype (A/J) and H-2d haplotype (BALB/c, DBA/2) did not. In the area of acute focal inflammation in mice with the H-2b and H-2k haplotypes, tachyzoites and toxoplasma antigens were demonstrated by immunoperoxidase staining, suggesting that the focal inflammation was induced by toxoplasma organisms. B10 congenic mice were used for further analysis of this genetic regulation. Presence of the encephalitis in B10 and B10.BR but not in B10.A and B10.D2 mice at 10 weeks after infection indicated regulation of the inflammation by a gene(s) within the H-2 complex. The encephalitis developed in B10.A (2R) and B10.A (4R) mice but not in B10.A (3R) and B10.A (18R) during infection. These results clearly indicated that the development of toxoplasmic encephalitis was controlled by a gene(s) in the H-2D region. The Qa and Tla genes did not appear to be critical in determining susceptibility to the encephalitis. There was no correlation between serum toxoplasma antibody titres and occurrence of the encephalitis. Injection of a monoclonal antibody to interferon-gamma (IFN-gamma) remarkably augmented the inflammatory changes in the brains of the infected B10 mice. In contrast, the treatment did not induce any inflammatory response in the brains of the infected BALB/c mice. A similar genetic regulation may be operative in determining development of toxoplasmic encephalitis in AIDS and other immunocompromised patients.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Williams D. M., Grumet F. C., Remington J. S. Strain-dependent differences in murine susceptibility to toxoplasma. Infect Immun. 1976 May;13(5):1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Remington J. S., Sharma S. D. Protective immunity in toxoplasmosis: correlation between antibody response, brain cyst formation, T-cell activation, and survival in normal and B-cell-deficient mice bearing the H-2k haplotype. Infect Immun. 1987 Apr;55(4):990–994. doi: 10.1128/iai.55.4.990-994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Sharma S. D., Remington J. S. Different regulation of the L3T4-T cell subset by B cells in different mouse strains bearing the H-2k haplotype. J Immunol. 1986 Nov 1;137(9):2991–2997. [PubMed] [Google Scholar]
- Brown C. R., McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990 Nov 15;145(10):3438–3441. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley F. K., Jenkins K. A., Remington J. S. Toxoplasma gondii infection of the central nervous system. Use of the peroxidase-antiperoxidase method to demonstrate toxoplasma in formalin fixed, paraffin embedded tissue sections. Hum Pathol. 1981 Aug;12(8):690–698. doi: 10.1016/s0046-8177(81)80170-0. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Hutchison W. M., Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. An immunocytochemical and ultrastructural study. Parasitol Res. 1989;75(8):599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Conley F. K., Remington J. S. Murine model of intracerebral toxoplasmosis. J Infect Dis. 1987 Mar;155(3):550–557. doi: 10.1093/infdis/155.3.550. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Erb P. H-2 complex-linked resistance in murine toxoplasmosis. J Infect Dis. 1985 Apr;151(4):739–740. doi: 10.1093/infdis/151.4.739. [DOI] [PubMed] [Google Scholar]
- Khan I. A., Smith K. A., Kasper L. H. Induction of antigen-specific human cytotoxic T cells by Toxoplasma gondii. J Clin Invest. 1990 Jun;85(6):1879–1886. doi: 10.1172/JCI114649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. AIDS commentary. Toxoplasmic encephalitis. J Infect Dis. 1988 Jan;157(1):1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989 Sep 15;143(6):2045–2050. [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol. 1988 Jun 1;140(11):3943–3946. [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990 Mar 1;144(5):1954–1956. [PubMed] [Google Scholar]