Abstract
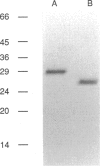
Faecally enriched extracts of Dermatophagoides pteronyssinus were shown to contain a trypsin-like enzyme which was allergenic. Chromatofocusing studies revealed the presence of nine major isoforms in D. pteronyssinus, with pI in the range 4 to greater than 8, but only two (range 4-5) in D. farinae. Trypsin isolated from D. pteronyssinus by benzamidine-Sepharose 6B affinity chromatography and gelfiltration was found to be a 31-kDa protein which was enzymatically similar to both invertebrate and vertebrate trypsins. The N-terminal sequence obtained (IVGGEXALAGEXPYQISL) was identical to that reported for the mite allergen Der p III and showed homology with crayfish trypsin and Der f III from D. farinae. Mite trypsin underwent autolysis and the N-terminal sequences of two fragments were found to be ALAGEXPYQI and NNQVXGI respectively. Both showed homology with crayfish trypsin, and the former sequence was identical to residues 7-18 of the native enzyme and Der p III. All isoforms of mite trypsin were showed to be allergenic by radioallergosorbent assay and further studies indicated that the trypsin degradation products were also allergenic. The enzyme was compared with other mite allergens and the rank order of allergenic potency was shown to be: whole mite extract greater than Der p I greater than trypsin. However, all sera from a panel of mite allergic individuals showed IgE reactivity to trypsin, comparable to that seen using whole mite extract and Der p I. These data indicate that mite trypsin is a major allergen corresponding to the previously described allergen, Der p III.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman M. D., Platts-Mills T. A. Measurement of IgG, IgA and IgE antibodies to Dermatophagoides pteronyssinus by antigen-binding assay, using a partially purified fraction of mite extract (F4P1). Clin Exp Immunol. 1978 Oct;34(1):126–136. [PMC free article] [PubMed] [Google Scholar]
- Chavira R., Jr, Burnett T. J., Hageman J. H. Assaying proteinases with azocoll. Anal Biochem. 1984 Feb;136(2):446–450. doi: 10.1016/0003-2697(84)90242-2. [DOI] [PubMed] [Google Scholar]
- Chua K. Y., Stewart G. A., Thomas W. R., Simpson R. J., Dilworth R. J., Plozza T. M., Turner K. J. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J Exp Med. 1988 Jan 1;167(1):175–182. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S. A., Tovey E. R., Baldo B. A. The spectrum of low molecular weight house dust mite (Dermatophagoides pteronyssinus) allergens with emphasis on Der p II. Clin Exp Allergy. 1990 Jan;20(1):27–31. doi: 10.1111/j.1365-2222.1990.tb02771.x. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Heymann P. W., Chapman M. D., Aalberse R. C., Fox J. W., Platts-Mills T. A. Antigenic and structural analysis of group II allergens (Der f II and Der p II) from house dust mites (Dermatophagoides spp). J Allergy Clin Immunol. 1989 Jun;83(6):1055–1067. doi: 10.1016/0091-6749(89)90447-8. [DOI] [PubMed] [Google Scholar]
- Ino Y., Ando T., Haida M., Nakamura K., Iwaki M., Okudaira H., Miyamoto T. Characterization of the proteases in the crude mite extract. Int Arch Allergy Appl Immunol. 1989;89(4):321–326. doi: 10.1159/000234970. [DOI] [PubMed] [Google Scholar]
- Krilis S., Baldo B. A., Basten A. Dialyzable allergens from the common house dust mites. Naturwissenschaften. 1979 Sep;66(9):475–476. doi: 10.1007/BF00399012. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake F. R., Ward L. D., Simpson R. J., Thompson P. J., Stewart G. A. House dust mite-derived amylase: allergenicity and physicochemical characterization. J Allergy Clin Immunol. 1991 Jun;87(6):1035–1042. doi: 10.1016/0091-6749(91)92147-s. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Shaw E. Pseudotrypsin. A modified bovine trypsin produced by limited autodigestion. J Biol Chem. 1969 Sep 10;244(17):4704–4712. [PubMed] [Google Scholar]
- Stewart G. A. Isolation and characterization of the allergen Dpt 12 from Dermatophagoides pteronyssinus by chromatofocusing. Int Arch Allergy Appl Immunol. 1982;69(3):224–230. doi: 10.1159/000233175. [DOI] [PubMed] [Google Scholar]
- Stewart G. A., Thompson P. J., Simpson R. J. Protease antigens from house dust mite. Lancet. 1989 Jul 15;2(8655):154–155. doi: 10.1016/s0140-6736(89)90203-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Aoki T., Kohmoto S., Nishimura H., Kodera Y., Matsushima A., Inada Y. Activation of kallikrein-kinin system in human plasma with purified serine protease from Dermatophagoides farinae. Int Arch Allergy Appl Immunol. 1990;91(1):80–85. doi: 10.1159/000235094. [DOI] [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Woodbury R. G., Ericsson L. H., Dörsam H., Kraemer M., Neurath H., Zwilling R. Amino acid sequence of crayfish (Astacus fluviatilis) trypsin If. Biochemistry. 1983 Mar 15;22(6):1459–1465. doi: 10.1021/bi00275a021. [DOI] [PubMed] [Google Scholar]
- Ward L. D., Hong J., Whitehead R. H., Simpson R. J. Development of a database of amino acid sequences for human colon carcinoma proteins separated by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1990 Oct;11(10):883–891. doi: 10.1002/elps.1150111019. [DOI] [PubMed] [Google Scholar]
- van Toorenenbergen A. W., Huijskes-Heins M. I., Dieges P. H., Leijnse B. Occupational allergy to pancreatic powder: characterization of IgE-binding antigens in pancreatic extract by immunoblotting. J Allergy Clin Immunol. 1991 Mar;87(3):650–654. doi: 10.1016/0091-6749(91)90383-y. [DOI] [PubMed] [Google Scholar]