Abstract
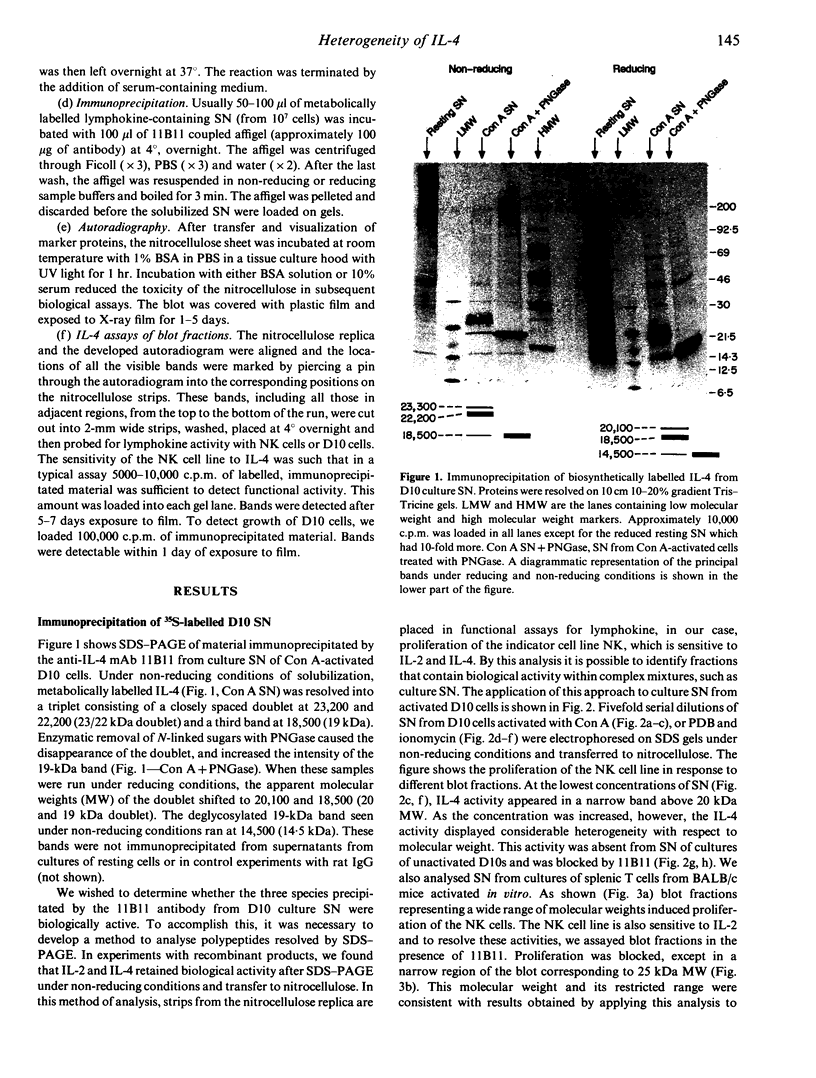
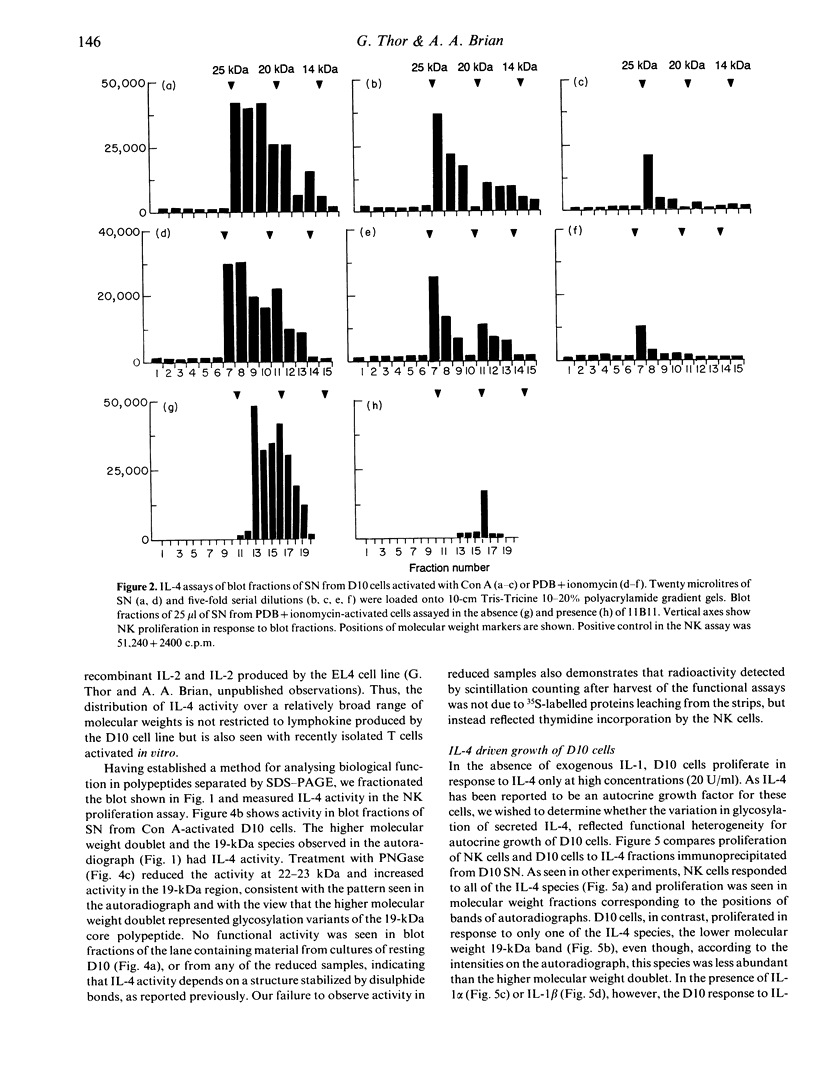
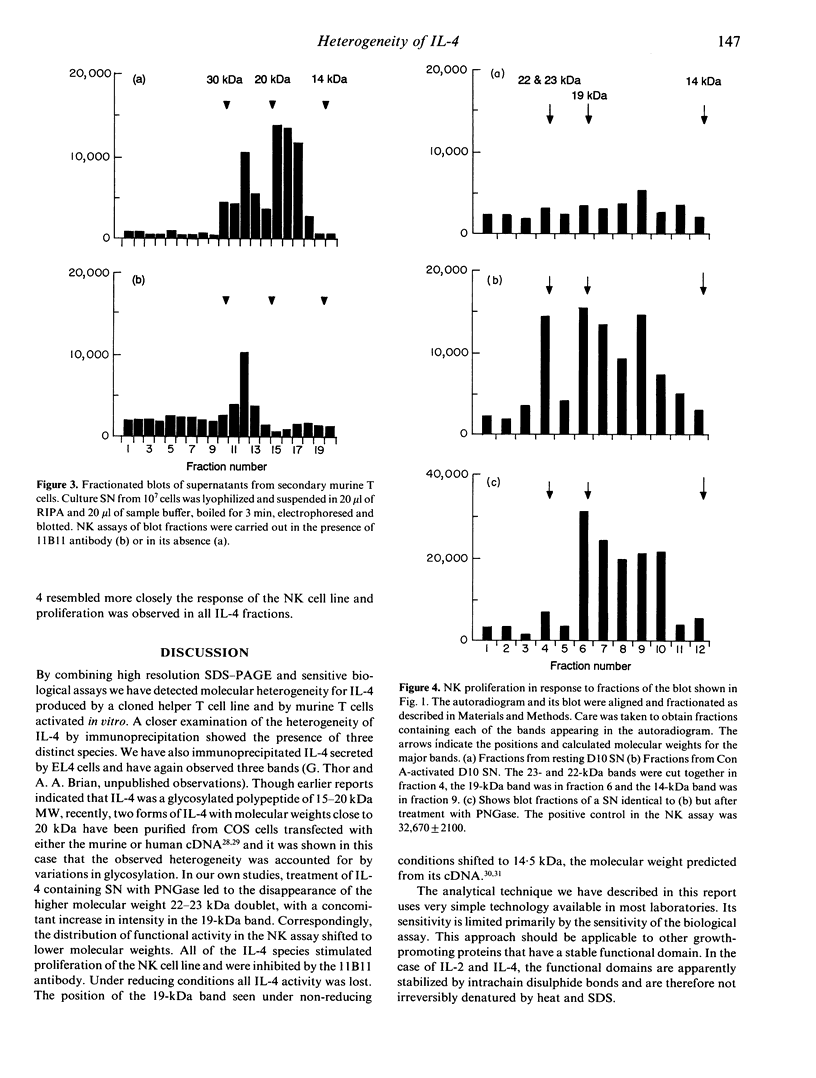
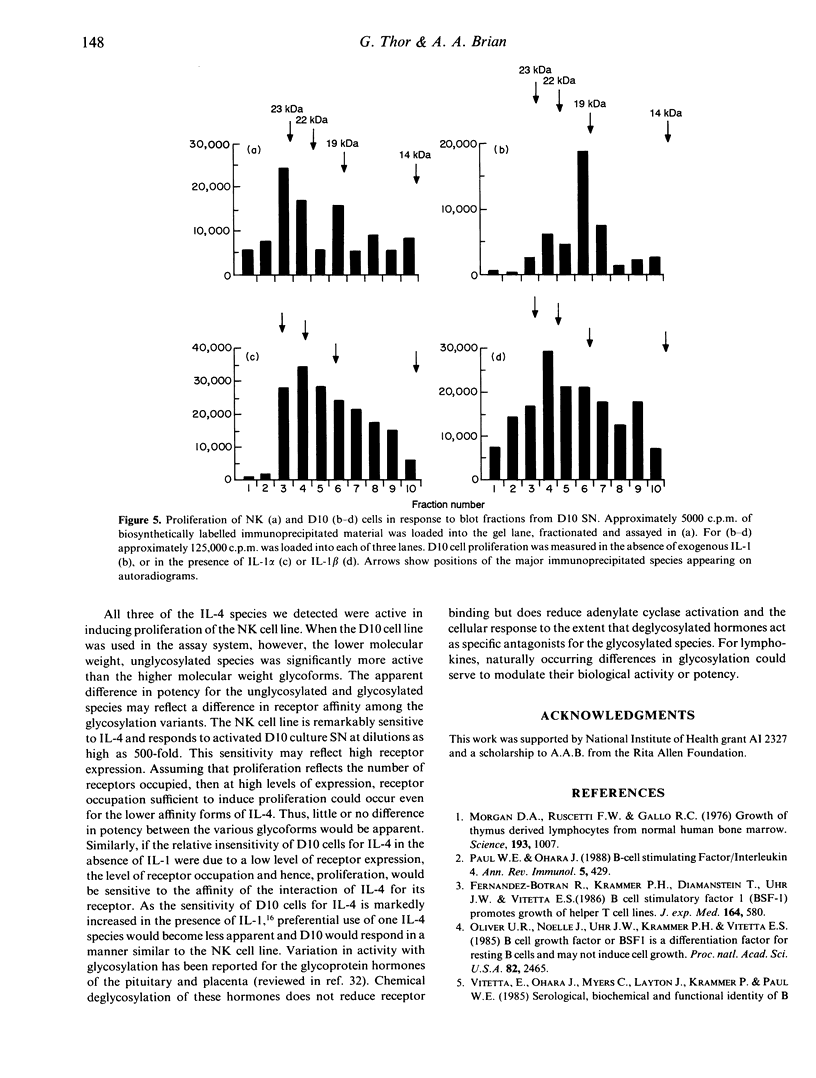
Immunoprecipitation of biosynthetically labelled interleukin-4 (IL-4) secreted by activated D10 cells yielded three bands after separation under non-reducing conditions by electrophoresis through gradient SDS polyacrylamide gels. This triplet consists of a closely spaced doublet at 23 and 22 kDa (23/22 kDa), and a third band at 18.5 kDa (19 kDa). The 23/22 kDa doublet was converted to the 19 kDa form by enzymatic removal of the N-linked sugars, indicating that the two glycoforms were derived from the 19-kDa core polypeptide. Under reducing conditions, the 19-kDa polypeptide migrated at 14.5 kDa, consistent with the size predicted from the complementary DNA (cDNA). Under non-reducing conditions, IL-4 retained biological activity after electrophoresis and transfer to nitrocellulose. Applying a biological assay, proliferation of the NK cell line, to fractionated nitrocellulose replicas, we found that IL-4 activity was detected over a relatively broad range of molecular weights, reflecting the multiple bands found by immunoprecipitation. This was true not only for IL-4 produced by D10 cells but also for splenic cells activated in vitro. All of the immunoprecipitated IL-4 species were active in inducing proliferation of the NK cell line. However, when the D10 cell line was used to detect IL-4, the 19-kDa species was significantly more active than the higher molecular weight species. These results suggest that different forms of IL-4 may have different functional properties.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S., Elder J. H. Endoglycosidases from Flavobacterium meningosepticum application to biological problems. Methods Enzymol. 1989;179:505–518. doi: 10.1016/0076-6879(89)79151-5. [DOI] [PubMed] [Google Scholar]
- Dennert G. Cloned lines of natural killer cells. Nature. 1980 Sep 4;287(5777):47–49. doi: 10.1038/287047a0. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T., Horowitz M., Lee F., Robb R., Flood P. M. Autocrine growth of T cells independent of interleukin 2: identification of interleukin 4 (IL 4, BSF-1) as an autocrine growth factor for a cloned antigen-specific helper T cell. J Immunol. 1987 Jun 15;138(12):4280–4287. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le H. V., Ramanathan L., Labdon J. E., Mays-Ichinco C. A., Syto R., Arai N., Hoy P., Takebe Y., Nagabhushan T. L., Trotta P. P. Isolation and characterization of multiple variants of recombinant human interleukin 4 expressed in mammalian cells. J Biol Chem. 1988 Aug 5;263(22):10817–10823. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Oliver K., Noelle R. J., Uhr J. W., Krammer P. H., Vitetta E. S. B-cell growth factor (B-cell growth factor I or B-cell-stimulating factor, provisional 1) is a differentiation factor for resting B cells and may not induce cell growth. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2465–2467. doi: 10.1073/pnas.82.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., McKenzie D. T., Swain S. L., Dutton R. W. B cell stimulatory factor 1 (interleukin 4) is sufficient for the proliferation and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Exp Med. 1987 Nov 1;166(5):1464–1470. doi: 10.1084/jem.166.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D., Yang G., Muller-Sieburg C., Smith C., Arai N., Takabe Y., Gemmell L. Interleukin 4 (B-cell stimulatory factor 1) can enhance or antagonize the factor-dependent growth of hemopoietic progenitor cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6889–6893. doi: 10.1073/pnas.84.19.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam M. R. Role of carbohydrates in glycoprotein hormone signal transduction. FASEB J. 1989 Jun;3(8):1915–1926. doi: 10.1096/fasebj.3.8.2542111. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Swain S. L., McKenzie D. T., Weinberg A. D., Hancock W. Characterization of T helper 1 and 2 cell subsets in normal mice. Helper T cells responsible for IL-4 and IL-5 production are present as precursors that require priming before they develop into lymphokine-secreting cells. J Immunol. 1988 Nov 15;141(10):3445–3455. [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Tartakovsky B., Finnegan A., Muegge K., Brody D. T., Kovacs E. J., Smith M. R., Berzofsky J. A., Young H. A., Durum S. K. IL-1 is an autocrine growth factor for T cell clones. J Immunol. 1988 Dec 1;141(11):3863–3867. [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]