Abstract
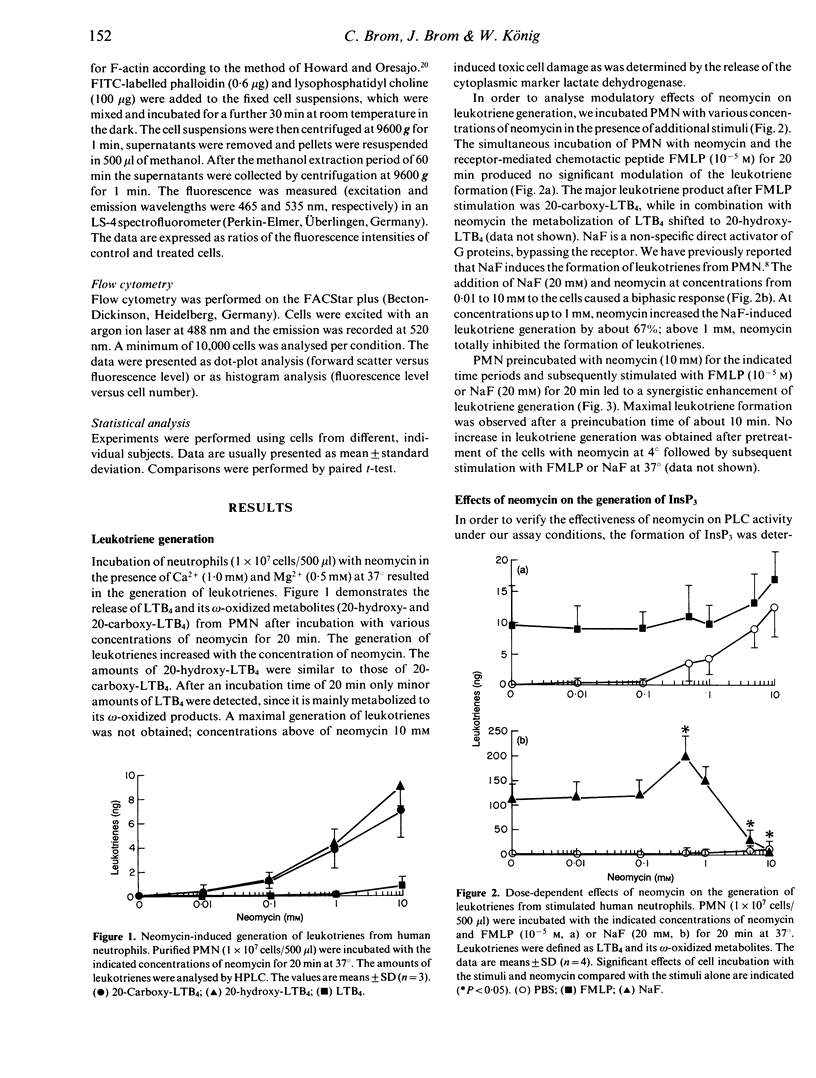
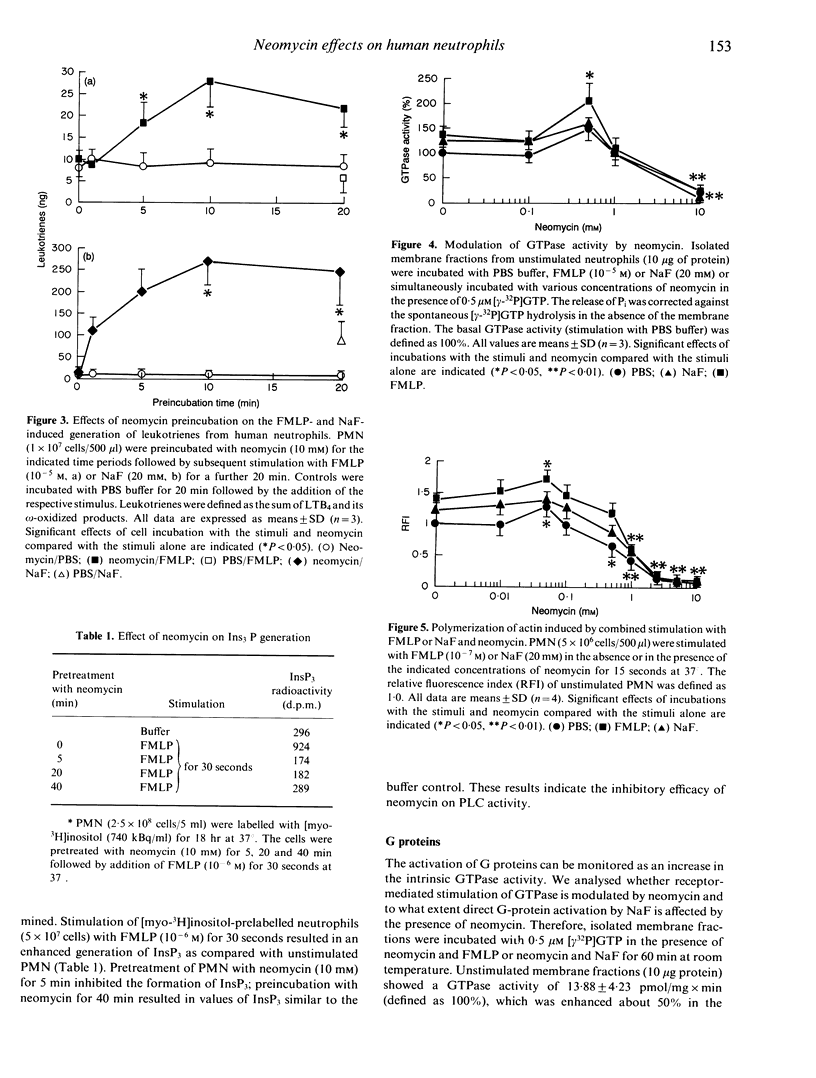
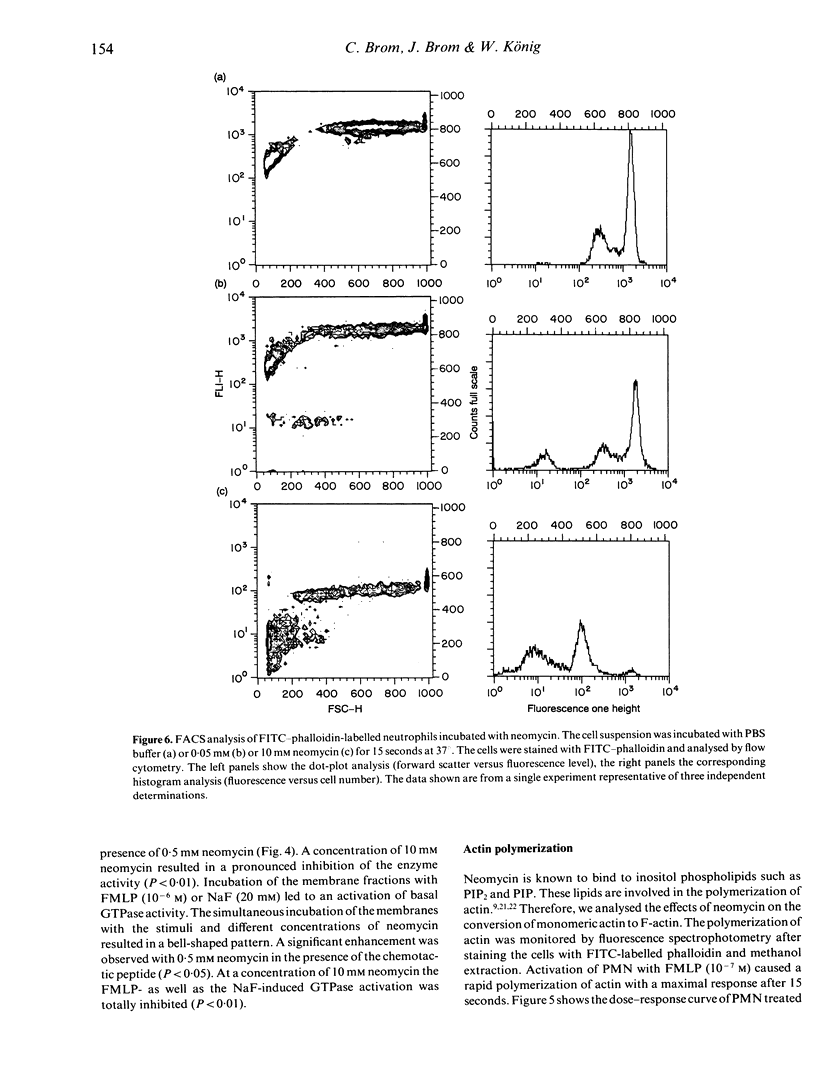
The effects of neomycin on human neutrophils (PMN) were studied with respect to the generation of leukotrienes, the involvement of guanine triphosphate binding proteins (G proteins) and the polymerization of actin. Incubation of neutrophils with neomycin induced the generation of low amounts of leukotrienes. Co-incubation of neutrophils with neomycin and the direct G-protein activator sodium fluoride (NaF) resulted in an enhanced leukotriene formation at 0.5 mM neomycin and an inhibition at a concentration of 10 mM. Simultaneous incubation with neomycin and formyl-methionyl-leucyl-phenylalanine (FLMP) did not affect the FMLP-induced leukotriene formation. However, pretreatment of neutrophils with 10 mM neomycin followed by the addition of NaF or FMLP resulted in an enhanced generation of leukotrienes. Crude membrane fractions of PMN incubated with neomycin at different concentrations showed an enhanced (0.5 mM) as well as a reduced (10 mM) guanine triphosphatase activity. Furthermore, incubation of neutrophils with neomycin above a concentration of 0.5 mM led to the depolymerization of actin. The presented results show inhibitory and stimulatory effects of neomycin on various cell functions, which may reflect differences in transmembrane signalling.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bray M. A. Leukotrienes in inflammation. Agents Actions. 1986 Oct;19(1-2):87–99. doi: 10.1007/BF01977263. [DOI] [PubMed] [Google Scholar]
- Brom C., Köller M., Brom J., König W. Effect of sodium fluoride on the generation of lipoxygenase products from human polymorphonuclear granulocytes, mononuclear cells and platelets--indication for the involvement of G proteins. Immunology. 1989 Oct;68(2):240–246. [PMC free article] [PubMed] [Google Scholar]
- Burn P. Amphitropic proteins: a new class of membrane proteins. Trends Biochem Sci. 1988 Mar;13(3):79–83. doi: 10.1016/0968-0004(88)90043-6. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. Effect of pertussis toxin and neomycin on G-protein-regulated polyphosphoinositide phosphodiesterase. A comparison between HL60 membranes and permeabilized HL60 cells. Biochem J. 1988 Dec 1;256(2):343–350. doi: 10.1042/bj2560343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bianca V., Grzeskowiak M., Dusi S., Rossi F. Fluoride can activate the respiratory burst independently of Ca2+, stimulation of phosphoinositide turnover and protein kinase C translocation in primed human neutrophils. Biochem Biophys Res Commun. 1988 Feb 15;150(3):955–964. doi: 10.1016/0006-291x(88)90722-x. [DOI] [PubMed] [Google Scholar]
- Dewald B., Payne T. G., Baggiolini M. Activation of NADPH oxidase of human neutrophils. Potentiation of chemotactic peptide by a diacylglycerol. Biochem Biophys Res Commun. 1984 Nov 30;125(1):367–373. doi: 10.1016/s0006-291x(84)80377-0. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G. P., Chan C. K., Grinstein S. Actin assembly in electropermeabilized neutrophils: role of G-proteins. Biochem Biophys Res Commun. 1989 Oct 31;164(2):700–705. doi: 10.1016/0006-291x(89)91516-7. [DOI] [PubMed] [Google Scholar]
- Gabev E., Kasianowicz J., Abbott T., McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta. 1989 Feb 13;979(1):105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- Herrmann E., Jakobs K. H. Stimulation and inhibition of human platelet membrane high-affinity GTPase by neomycin. FEBS Lett. 1988 Feb 29;229(1):49–53. doi: 10.1016/0014-5793(88)80795-6. [DOI] [PubMed] [Google Scholar]
- Howard T. H., Oresajo C. O. The kinetics of chemotactic peptide-induced change in F-actin content, F-actin distribution, and the shape of neutrophils. J Cell Biol. 1985 Sep;101(3):1078–1085. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Lefer A. M. Leukotrienes as mediators of ischemia and shock. Biochem Pharmacol. 1986 Jan 15;35(2):123–127. doi: 10.1016/0006-2952(86)90502-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Molski T. F., Kanaho Y., Becker E. L., Sha'afi R. I. G-protein dissociation, GTP-GDP exchange and GTPase activity in control and PMA treated neutrophils stimulated by fMet-Leu-Phe. Biochem Biophys Res Commun. 1987 Mar 13;143(2):489–498. doi: 10.1016/0006-291x(87)91380-5. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Tohmatsu T., Shirato L., Takenaka A., Nozawa Y. Neomycin is a potent agent for arachidonic acid release in human platelets. Biochem Biophys Res Commun. 1987 Jul 31;146(2):820–826. doi: 10.1016/0006-291x(87)90604-8. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Schmitt J. D., McCall C. E., Wykle R. L. Diacylglycerols enhance human neutrophil degranulation responses: relevancy to a multiple mediator hypothesis of cell function. Biochem Biophys Res Commun. 1984 Aug 30;123(1):64–70. doi: 10.1016/0006-291x(84)90380-2. [DOI] [PubMed] [Google Scholar]
- Prentki M., Deeney J. T., Matschinsky F. M., Joseph S. K. Neomycin: a specific drug to study the inositol-phospholipid signalling system? FEBS Lett. 1986 Mar 3;197(1-2):285–288. doi: 10.1016/0014-5793(86)80343-x. [DOI] [PubMed] [Google Scholar]
- Sastrasinh M., Knauss T. C., Weinberg J. M., Humes H. D. Identification of the aminoglycoside binding site in rat renal brush border membranes. J Pharmacol Exp Ther. 1982 Aug;222(2):350–358. [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Neomycin inhibits inositol phosphate formation in human platelets stimulated by thrombin but not other agonists. FEBS Lett. 1986 Oct 20;207(1):53–57. doi: 10.1016/0014-5793(86)80011-4. [DOI] [PubMed] [Google Scholar]
- Strnad C. F., Wong K. Calcium mobilization in fluoride activated human neutrophils. Biochem Biophys Res Commun. 1985 Nov 27;133(1):161–167. doi: 10.1016/0006-291x(85)91855-8. [DOI] [PubMed] [Google Scholar]
- Stumpo R. J., Pullan L. M., Salama A. I. The inhibition of [125I]omega-conotoxin GVIA binding to neuronal membranes by neomycin may be mediated by a GTP-binding protein. Eur J Pharmacol. 1991 Feb 25;206(2):155–158. doi: 10.1016/0922-4106(91)90024-c. [DOI] [PubMed] [Google Scholar]
- Särndahl E., Lindroth M., Bengtsson T., Fällman M., Gustavsson J., Stendahl O., Andersson T. Association of ligand-receptor complexes with actin filaments in human neutrophils: a possible regulatory role for a G-protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2791–2799. doi: 10.1083/jcb.109.6.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien S., Naccache P. H. Guanine nucleotide-induced polymerization of actin in electropermeabilized human neutrophils. J Cell Biol. 1989 Sep;109(3):1125–1132. doi: 10.1083/jcb.109.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes O. B., Steen V. M., Holmsen H. Neomycin inhibits platelet functions and inositol phospholipid metabolism upon stimulation with thrombin, but not with ionomycin or 12-O-tetradecanoyl-phorbol 13-acetate. Eur J Biochem. 1988 Oct 15;177(1):219–223. doi: 10.1111/j.1432-1033.1988.tb14365.x. [DOI] [PubMed] [Google Scholar]


