Abstract
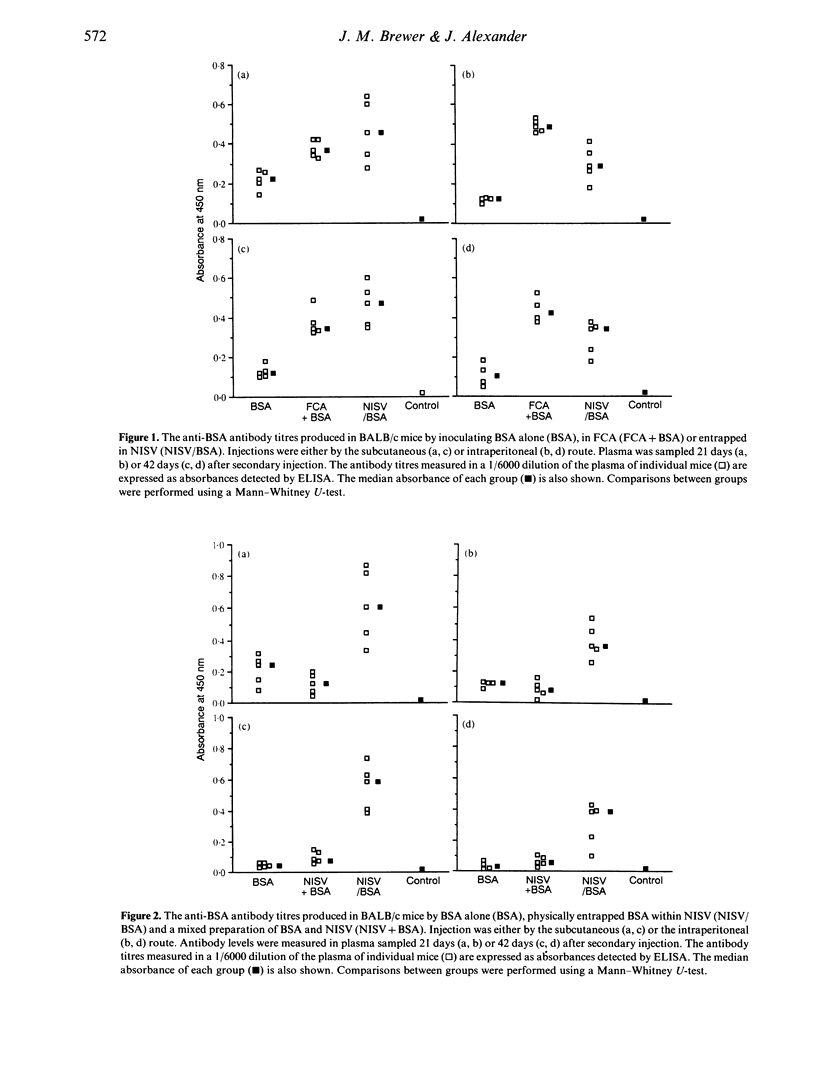
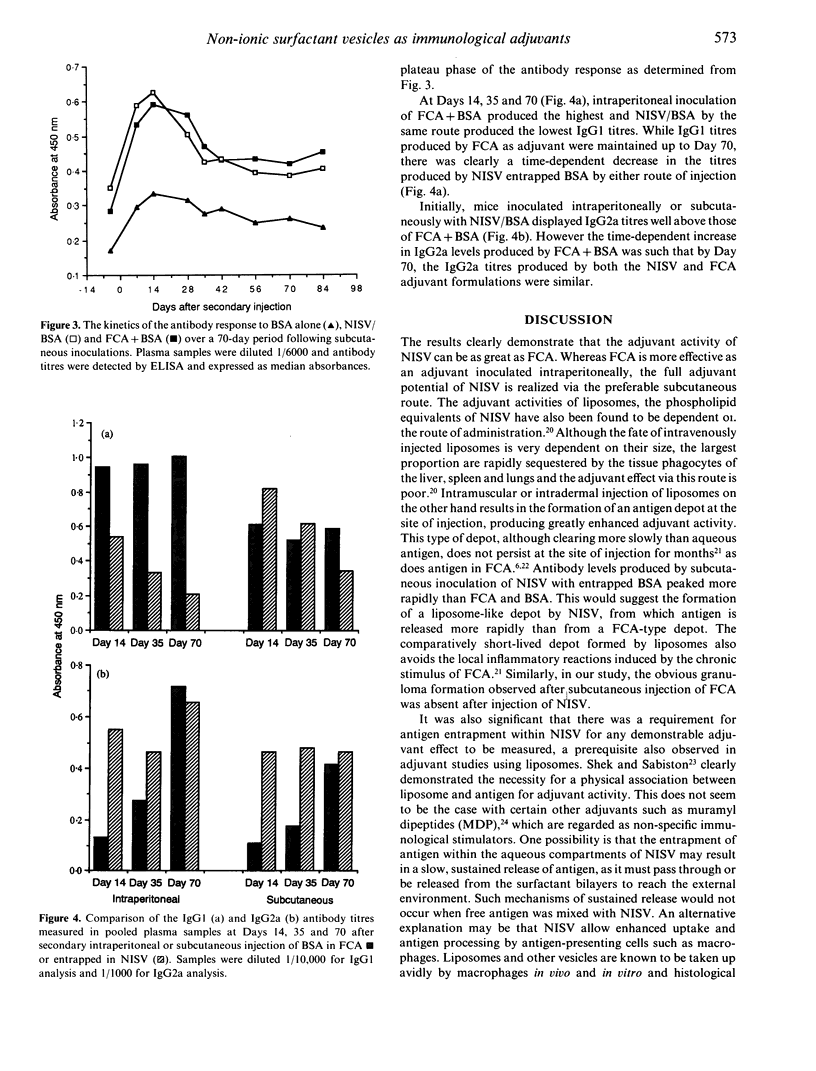
The ability of non-ionic surfactant vesicles (NISV) to enhance antibody production against bovine serum albumin (BSA) was compared with Freund's complete adjuvant (FCA), in the BALB/c mouse. Two subcutaneous inoculations with NISV entrapped BSA induced antibody levels comparable to, and persisting as long as those produced by FCA by either the subcutaneous or intraperitoneal route of inoculation. Intraperitoneal inoculation of NISV did not generate as strong an antibody response. The adjuvant activity of NISV was wholly dependent on the BSA being entrapped within preformed vesicles; mixing free BSA with vesicles was not effective. Analysis of the anti-BSA IgG subclasses induced by NISV and FCA showed that NISV were generally better stimulators of IgG2a than was FCA, but poorer stimulators of IgG1. From this, we deduce that NISV are potentially better stimulators of the Th1 lymphocyte subset than is FCA and by inference, potent stimulators of cellular immunity. We believe that NISV may offer many advantages over other adjuvants in terms of immunological selectivity, low toxicity and stability.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Azmin M. N., Florence A. T., Handjani-Vila R. M., Stuart J. F., Vanlerberghe G., Whittaker J. S. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J Pharm Pharmacol. 1985 Apr;37(4):237–242. doi: 10.1111/j.2042-7158.1985.tb05051.x. [DOI] [PubMed] [Google Scholar]
- Baillie A. J., Florence A. T., Hume L. R., Muirhead G. T., Rogerson A. The preparation and properties of niosomes--non-ionic surfactant vesicles. J Pharm Pharmacol. 1985 Dec;37(12):863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Bomford R. Adjuvants for anti-parasite vaccines. Parasitol Today. 1989 Feb;5(2):41–46. doi: 10.1016/0169-4758(89)90190-7. [DOI] [PubMed] [Google Scholar]
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980 Feb;39(2):435–441. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Dal Monte P., Szoka F. C., Jr Effect of liposome encapsulation on antigen presentation in vitro. Comparison of presentation by peritoneal macrophages and B cell tumors. J Immunol. 1989 Mar 1;142(5):1437–1443. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. The carrier potential of liposomes in biology and medicine (first of two parts). N Engl J Med. 1976 Sep 23;295(13):704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- Grun J. L., Maurer P. H. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989 Jun;121(1):134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Herbert W. J. The mode of action of mineral-oil emulsion adjuvants on antibody production in mice. Immunology. 1968 Mar;14(3):301–318. [PMC free article] [PubMed] [Google Scholar]
- Hunter R. L., Bennett B. The adjuvant activity of nonionic block polymer surfactants. II. Antibody formation and inflammation related to the structure of triblock and octablock copolymers. J Immunol. 1984 Dec;133(6):3167–3175. [PubMed] [Google Scholar]
- Hunter R., Strickland F., Kézdy F. The adjuvant activity of nonionic block polymer surfactants. I. The role of hydrophile-lipophile balance. J Immunol. 1981 Sep;127(3):1244–1250. [PubMed] [Google Scholar]
- Kramp W. J., Six H. R., Kasel J. A. Postimmunization clearance of liposome entrapped adenovirus type 5 hexon. Proc Soc Exp Biol Med. 1982 Jan;169(1):135–139. doi: 10.3181/00379727-169-41321. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc C., Vogel F. R. Synthetic immunomodulators and synthetic vaccines. Crit Rev Ther Drug Carrier Syst. 1986;2(4):353–406. [PubMed] [Google Scholar]
- Morein B., Sundquist B., Höglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. 1984 Mar 29-Apr 4Nature. 308(5958):457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Rogerson A., Cummings J., Willmott N., Florence A. T. The distribution of doxorubicin in mice following administration in niosomes. J Pharm Pharmacol. 1988 May;40(5):337–342. doi: 10.1111/j.2042-7158.1988.tb05263.x. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Shek P. N., Sabiston B. H. Immune response mediated by liposome-associated protein antigens. I. Potentiation of the plaque-forming cell response. Immunology. 1982 Feb;45(2):349–356. [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988 Jan 1;167(1):183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]



