Abstract
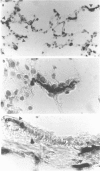
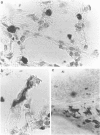
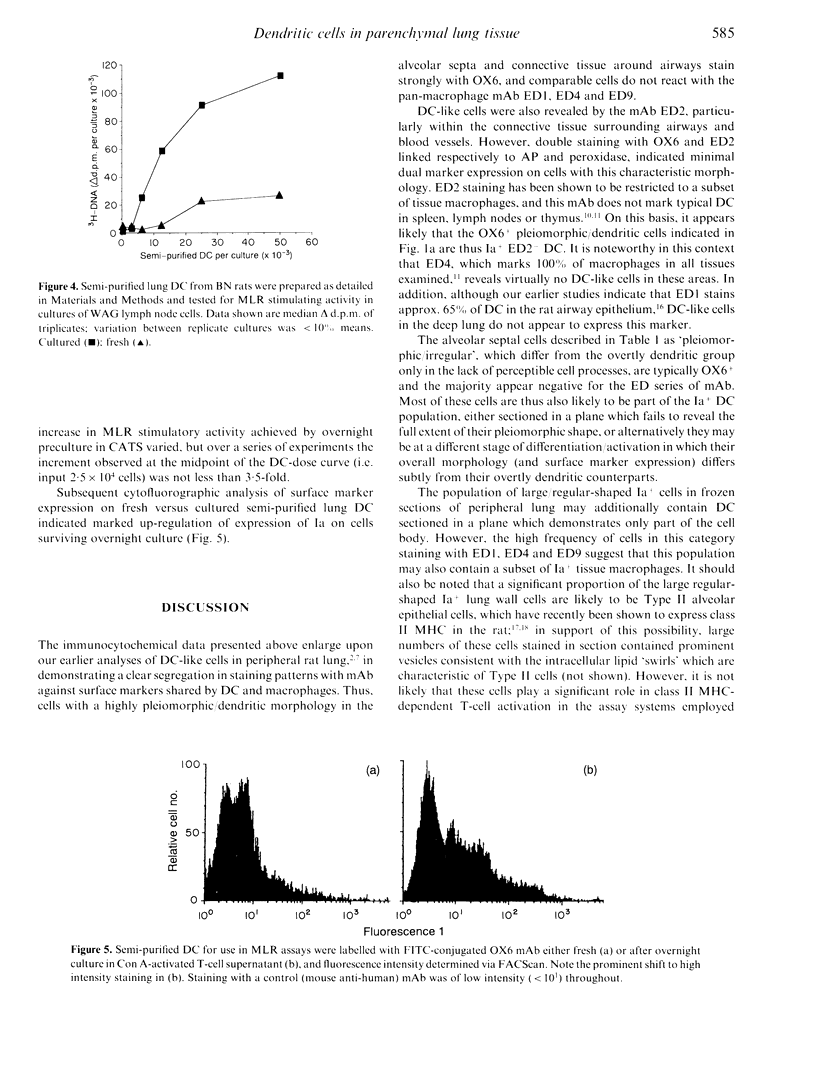
Immunohistochemical analysis of frozen sections of rat lung tissue identified a widely distributed population of highly pleiomorphic Ia+ cells in alveolar septal walls, which are negative for the pan-macrophage marker ED4 and the related markers ED1, ED2 and ED9. Semi-purified dendritic cells (DC) prepared from lungs of rats exposed to an aerosol of ovalbumin (OA) triggered modest levels of proliferation of OA-immune T cells in vitro, demonstrating the potential of these cells in surveillance for inhaled antigens in vivo. Lung wall also exhibited modest stimulatory activity in mixed lymphocyte/leucocyte reaction (MLR) assays. Overnight incubation of the DC in T-cell culture supernatant markedly increased their T-cell stimulatory properties, concomitant with increased expression of Ia. These results suggest that analogous to epidermal Langerhans' cells, lung wall DC can effectively bind inhaled antigens in situ, but require additional maturation/activation signals before they can efficiently present the antigen to T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barfoot R., Denham S., Gyure L. A., Hall J. G., Hobbs S. M., Jackson L. E., Robertson D. Some properties of dendritic macrophages from peripheral lymph. Immunology. 1989 Oct;68(2):233–239. [PMC free article] [PubMed] [Google Scholar]
- Claassen E., Adler L. T., Adler F. L. Double immunocytochemical staining for the in situ study of allotype distribution during an anti-trinitrophenyl immune response in chimeric rabbits. J Histochem Cytochem. 1986 Aug;34(8):989–994. doi: 10.1177/34.8.2426338. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. G., Döpp E. A., Neefjes J. J., Beelen R. H., Dijkstra C. D. Heterogeneity of macrophages in the rat evidenced by variability in determinants: two new anti-rat macrophage antibodies against a heterodimer of 160 and 95 kd (CD11/CD18). J Leukoc Biol. 1989 Dec;46(6):556–564. doi: 10.1002/jlb.46.6.556. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Harbeck R. J., Gegen N. W., Struhar D., Mason R. Class II molecules on rat alveolar type II epithelial cells. Cell Immunol. 1988 Jan;111(1):139–147. doi: 10.1016/0008-8749(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Schon-Hegrad M. A. Localization of T cells, macrophages and dendritic cells in rat respiratory tract tissue: implications for immune function studies. Immunology. 1987 Nov;62(3):349–356. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Schon-Hegrad M. A., Oliver J., Holt B. J., McMenamin P. G. A contiguous network of dendritic antigen-presenting cells within the respiratory epithelium. Int Arch Allergy Appl Immunol. 1990;91(2):155–159. doi: 10.1159/000235107. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Schon-Hegrad M. A., Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988 Feb 1;167(2):262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kradin R. L., McCarthy K. M., Gifford J., Schneeberger E. E. Antigen-independent binding of T-cells by dendritic cells and alveolar macrophages in the rat. Am Rev Respir Dis. 1989 Jan;139(1):207–211. doi: 10.1164/ajrccm/139.1.207. [DOI] [PubMed] [Google Scholar]
- MacPherson G. G. Properties of lymph-borne (veiled) dendritic cells in culture. I. Modulation of phenotype, survival and function: partial dependence on GM-CSF. Immunology. 1989 Sep;68(1):102–107. [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Pugh C. W., Webb M. The rat mixed lymphocyte reaction: roles of a dendritic cell in intestinal lymph and T-cell subsets defined by monoclonal antibodies. Immunology. 1981 Sep;44(1):75–87. [PMC free article] [PubMed] [Google Scholar]
- Metlay J. P., Puré E., Steinman R. M. Distinct features of dendritic cells and anti-Ig activated B cells as stimulators of the primary mixed leukocyte reaction. J Exp Med. 1989 Jan 1;169(1):239–254. doi: 10.1084/jem.169.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicod L. P., Lipscomb M. F., Weissler J. C., Lyons C. R., Albertson J., Toews G. B. Mononuclear cells in human lung parenchyma. Characterization of a potent accessory cell not obtained by bronchoalveolar lavage. Am Rev Respir Dis. 1987 Oct;136(4):818–823. doi: 10.1164/ajrccm/136.4.818. [DOI] [PubMed] [Google Scholar]
- Paine R., 3rd, Mody C. H., Chavis A., Spahr M. A., Turka L. A., Toews G. B. Alveolar epithelial cells block lymphocyte proliferation in vitro without inhibiting activation. Am J Respir Cell Mol Biol. 1991 Sep;5(3):221–229. doi: 10.1165/ajrcmb/5.3.221. [DOI] [PubMed] [Google Scholar]
- Pollard A. M., Lipscomb M. F. Characterization of murine lung dendritic cells: similarities to Langerhans cells and thymic dendritic cells. J Exp Med. 1990 Jul 1;172(1):159–167. doi: 10.1084/jem.172.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puré E., Inaba K., Crowley M. T., Tardelli L., Witmer-Pack M. D., Ruberti G., Fathman G., Steinman R. M. Antigen processing by epidermal Langerhans cells correlates with the level of biosynthesis of major histocompatibility complex class II molecules and expression of invariant chain. J Exp Med. 1990 Nov 1;172(5):1459–1469. doi: 10.1084/jem.172.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester C. L., Goodell E. M., Stoltenborg J. K., Bowers W. E. Dendritic cells from rat lung are potent accessory cells. Am Rev Respir Dis. 1988 Jul;138(1):121–128. doi: 10.1164/ajrccm/138.1.121. [DOI] [PubMed] [Google Scholar]
- Schon-Hegrad M. A., Oliver J., McMenamin P. G., Holt P. G. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991 Jun 1;173(6):1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sertl K., Takemura T., Tschachler E., Ferrans V. J., Kaliner M. A., Shevach E. M. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986 Feb 1;163(2):436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S., Caughman S. W., Sharrow S. O., Stephany D., Katz S. I. Enhanced antigen-presenting capacity of cultured Langerhans' cells is associated with markedly increased expression of Ia antigen. J Immunol. 1987 Oct 15;139(8):2551–2555. [PubMed] [Google Scholar]