Abstract
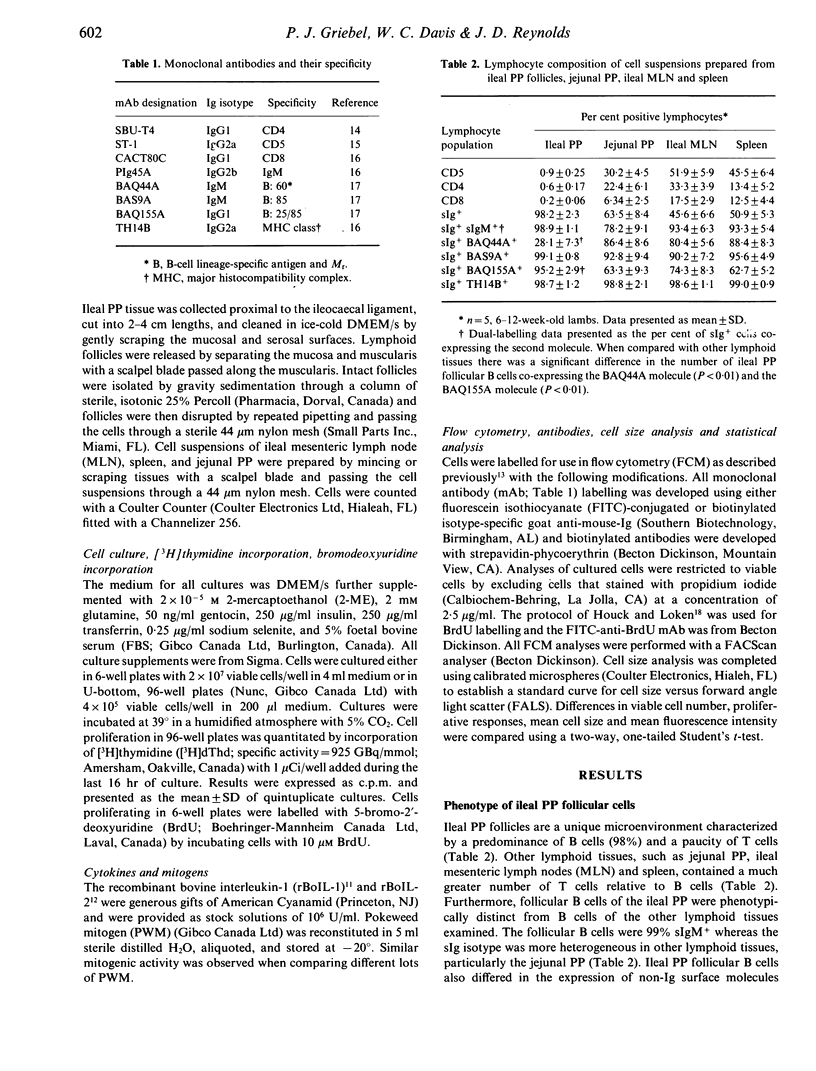
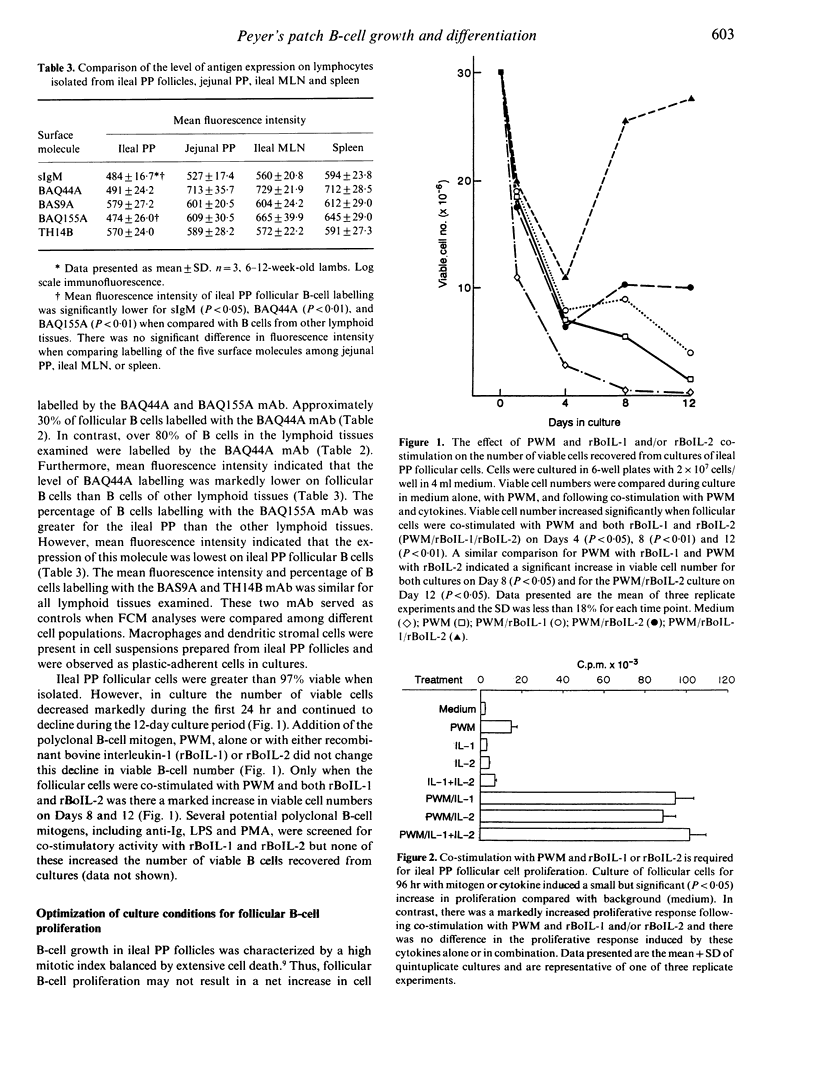
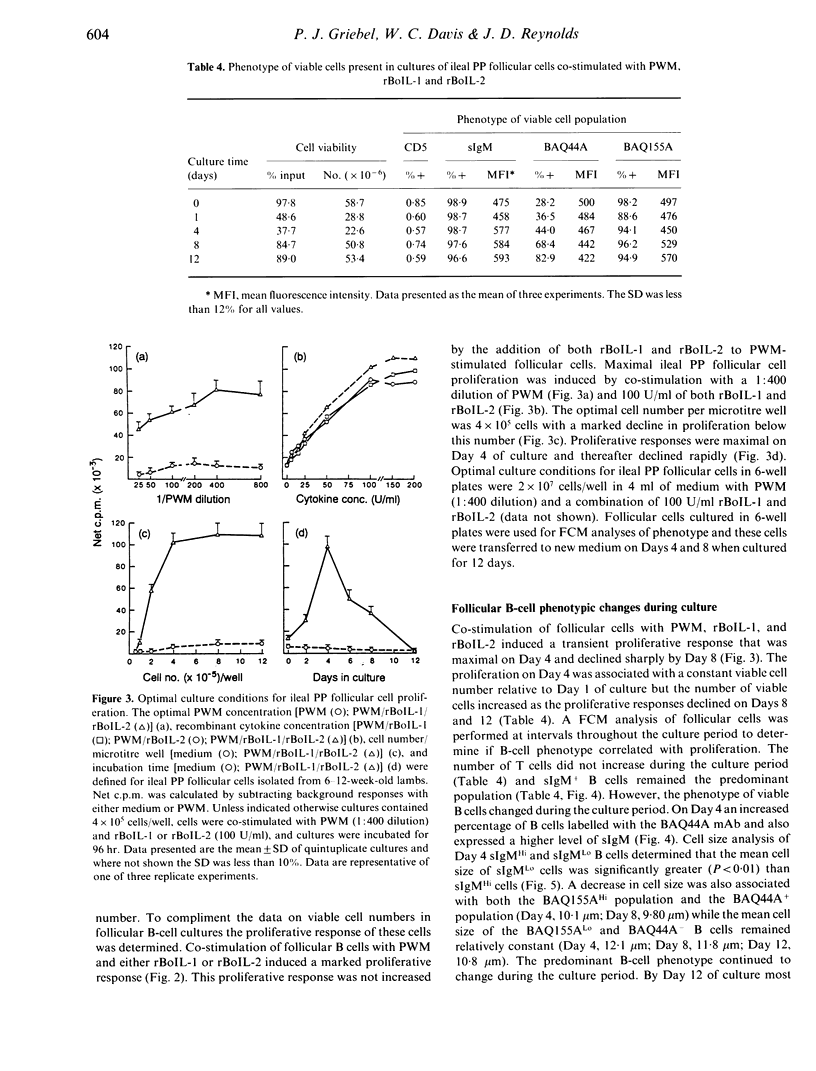
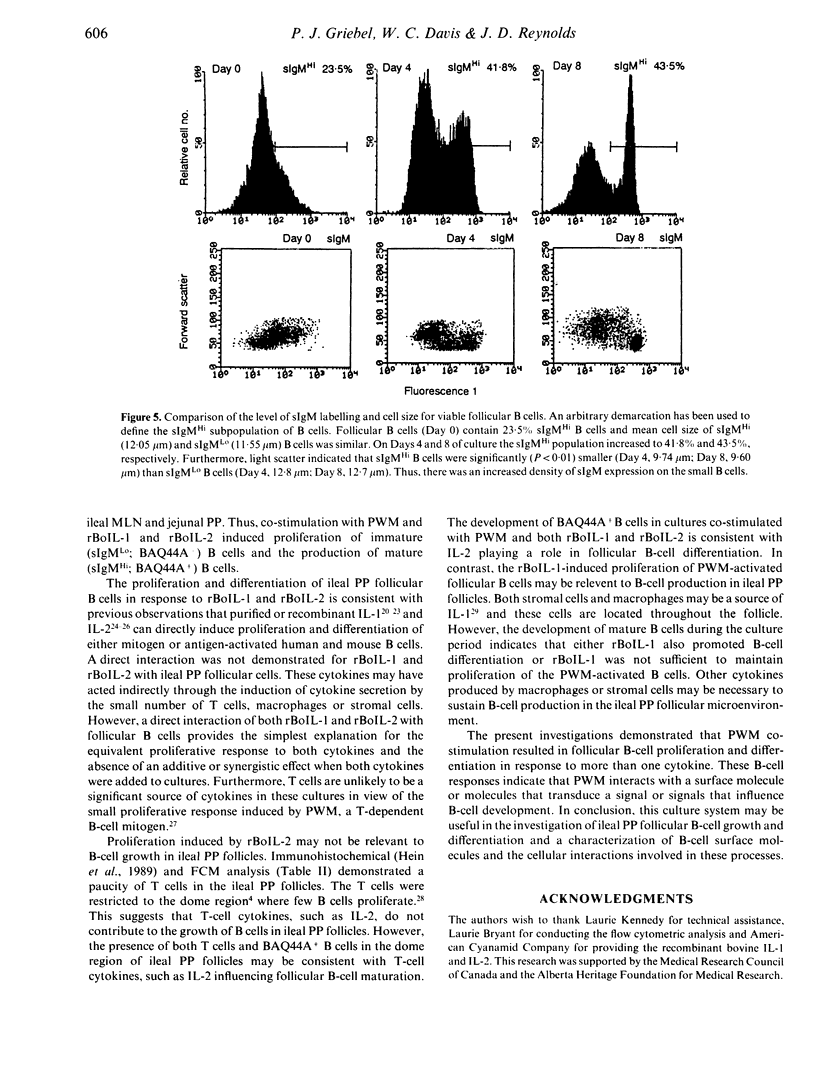
We developed a method to isolate and culture cells from the lymphoid follicles of the ileal Peyer's (PP) patch of young sheep (6-12 weeks). These cells were 98% sIgM+ B cells and 1% T cells. Cultured follicular cells were used to investigate B-cell proliferation and differentiation. Less than 50% of B cells were viable after 24 hr of culture and this decrease in B-cell viability also occurred following co-stimulation with pokeweed mitogen (PWM) and recombinant bovine interleukin-1 (rBoIL-1) or rBoIL-2. In contrast, co-stimulation with PWM and either rBoIL-1 or rBoIL-2 induced a marked proliferative response that was maximal on Day 4 of culture. Cytokine-induced proliferation of the B cells required PWM co-stimulation and proliferation induced by rBoIL-1 and rBoIL-2 was neither additive or synergistic. This suggests that PWM bound a molecule or molecules that signalled responsiveness to both rBoIL-1 and rBoIL-2. Culture of follicular cells with PWM and both rBoIL-1 and rBoIL-2 also resulted in B-cell differentiation. This differentiation was associated with decreased proliferation, an increased number of viable B cells, and increased expression of both surface IgM and non-Ig membrane molecules. Thus, co-stimulation of ileal PP follicular cells with PWM and rBoIL-1 and rBoIL-2 resulted in both B-cell proliferation and differentiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E. Bovine interleukin 2: cloning, high level expression, and purification. Vet Immunol Immunopathol. 1987 Dec;17(1-4):193–209. doi: 10.1016/0165-2427(87)90140-1. [DOI] [PubMed] [Google Scholar]
- Beya M. F., Miyasaka M., Dudler L., Ezaki T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. II. Two monoclonal antibodies that recognize all ovine T lymphocytes. Immunology. 1986 Jan;57(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- Chiplunkar S., Langhorne J., Kaufmann S. H. Stimulation of B cell growth and differentiation by murine recombinant interleukin 1. J Immunol. 1986 Dec 15;137(12):3748–3752. [PubMed] [Google Scholar]
- Gerber H. A., Morris B., Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986 Jun;64(Pt 3):201–213. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- Griebel P. J., Qualtiere L., Davis W. C., Lawman M. J., Babiuk L. A. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1987;1(4):267–286. doi: 10.1089/vim.1987.1.267. [DOI] [PubMed] [Google Scholar]
- Halleraker M., Landsverk T., Nicander L. Organization of ruminant Peyer's patches as seen with enzyme histochemical markers of stromal and accessory cells. Vet Immunol Immunopathol. 1990 Sep;26(1):93–104. doi: 10.1016/0165-2427(90)90135-f. [DOI] [PubMed] [Google Scholar]
- Houck D. W., Loken M. R. Simultaneous analysis of cell surface antigens, bromodeoxyuridine incorporation and DNA content. Cytometry. 1985 Nov;6(6):531–538. doi: 10.1002/cyto.990060607. [DOI] [PubMed] [Google Scholar]
- Howard M., Mizel S. B., Lachman L., Ansel J., Johnson B., Paul W. E. Role of interleukin 1 in anti-immunoglobulin-induced B cell proliferation. J Exp Med. 1983 May 1;157(5):1529–1543. doi: 10.1084/jem.157.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. Comparative activation requirements of human peripheral blood, spleen, and lymph node B cells. J Immunol. 1987 Aug 15;139(4):1005–1013. [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Larsen R. A., Monaghan M. L., Park Y. H., Hamilton M. J., Ellis J. A., Davis W. C. Identification and characterization of monoclonal antibodies reactive with bovine, caprine and ovine T-lymphocyte determinants by flow microfluorimetry. Vet Immunol Immunopathol. 1990 Jun;25(2):195–208. doi: 10.1016/0165-2427(90)90035-q. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Thompson P. A., Rosenwasser L. J., Dinarello C. A. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J Immunol. 1983 Jun;130(6):2708–2714. [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Maliszewski C. R., Baker P. E., Schoenborn M. A., Davis B. S., Cosman D., Gillis S., Cerretti D. P. Cloning, sequence and expression of bovine interleukin 1 alpha and interleukin 1 beta complementary DNAs. Mol Immunol. 1988 May;25(5):429–437. doi: 10.1016/0161-5890(88)90162-9. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., Dudler L., Bordmann G., Leiserson W. M., Gerber H. A., Reynolds J., Trnka Z. Differentiation of B lymphocytes in sheep. I. Phenotypic analysis of ileal Peyer's patch cells and the demonstration of a precursor population for sIg+ cells in the ileal Peyer's patches. Immunology. 1984 Nov;53(3):515–523. [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. Interleukin 1 can act as a B-cell growth and differentiation factor. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8153–8157. doi: 10.1073/pnas.82.23.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Raubitschek A., Nossal G. J. Human interleukin 2 can promote the growth and differentiation of single hapten-specific B cells in the presence of specific antigen. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7917–7921. doi: 10.1073/pnas.81.24.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Mackay C. R., Müller R. G., Weill J. C. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer's patches. Cell. 1991 Mar 8;64(5):995–1005. doi: 10.1016/0092-8674(91)90323-q. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D. Evidence of extensive lymphocyte death in sheep Peyer's patches. I. A comparison of lymphocyte production and export. J Immunol. 1986 Mar 15;136(6):2005–2010. [PubMed] [Google Scholar]
- Reynolds J. D. Mitotic rate maturation in the Peyer's patches of fetal sheep and in the bursa of Fabricius of the chick embryo. Eur J Immunol. 1987 Apr;17(4):503–507. doi: 10.1002/eji.1830170411. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The effect of antigen on the development of Peyer's patches in sheep. Eur J Immunol. 1984 Jan;14(1):1–6. doi: 10.1002/eji.1830140102. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The evolution and involution of Peyer's patches in fetal and postnatal sheep. Eur J Immunol. 1983 Aug;13(8):627–635. doi: 10.1002/eji.1830130805. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


