Abstract
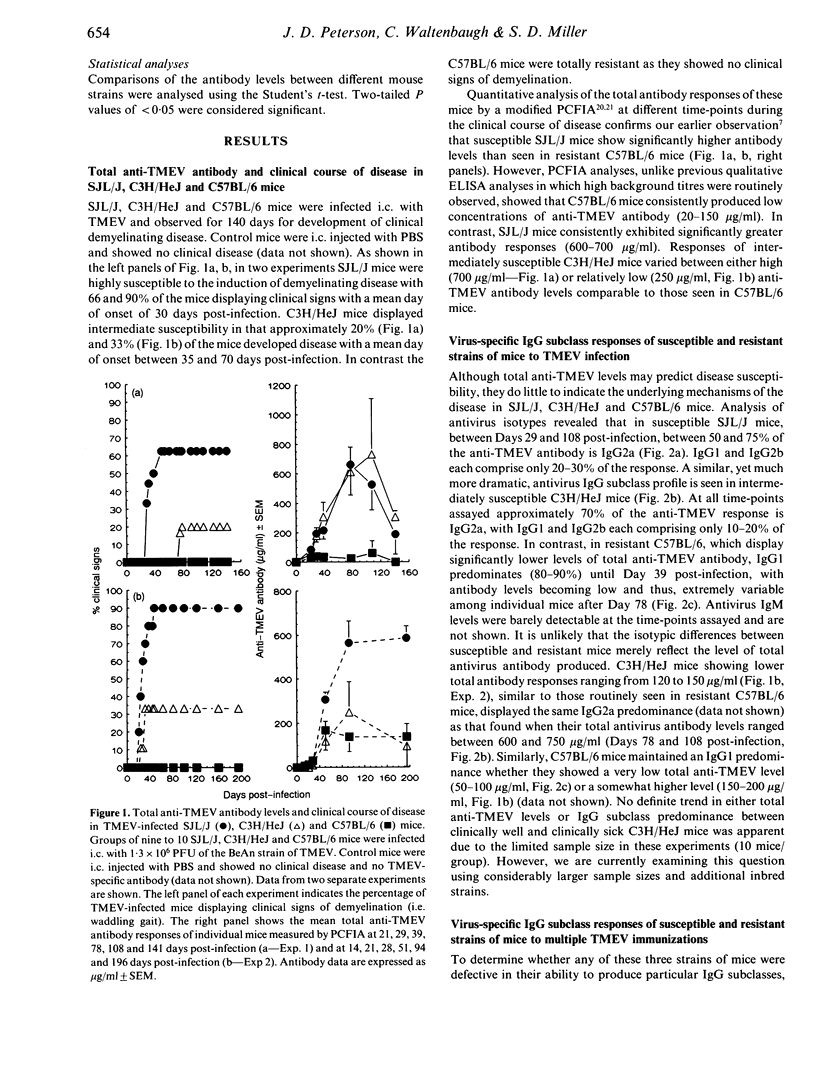
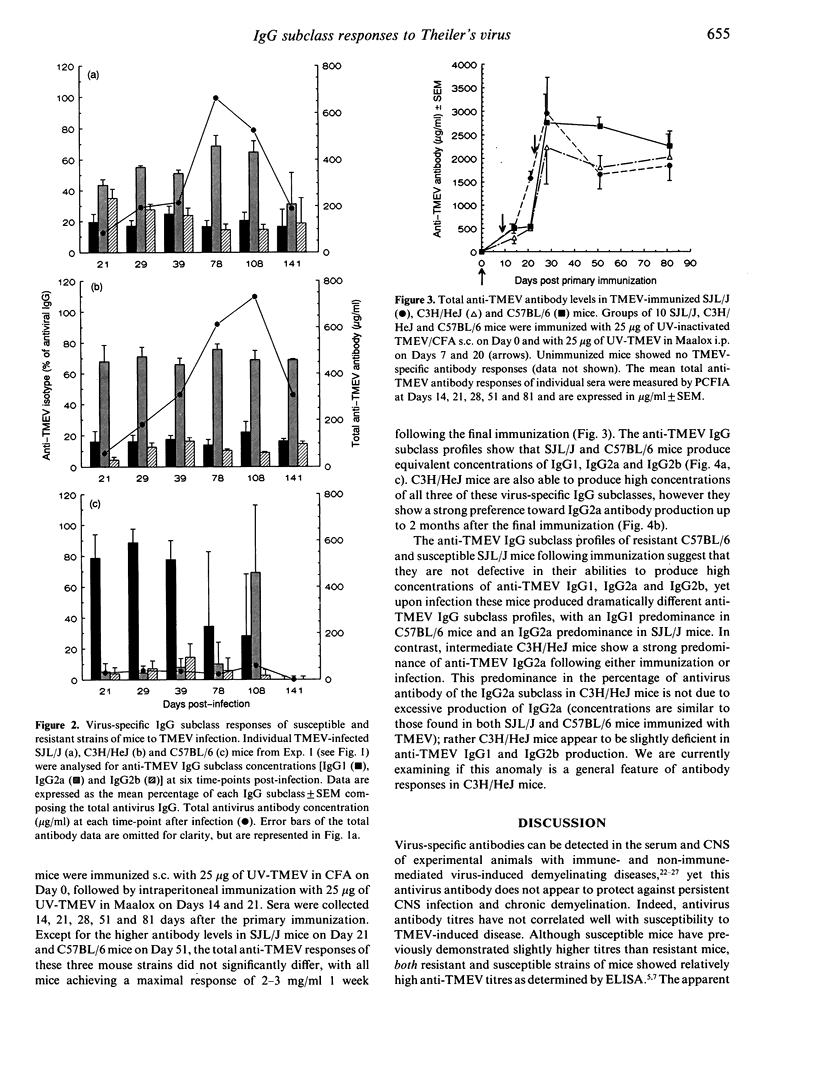
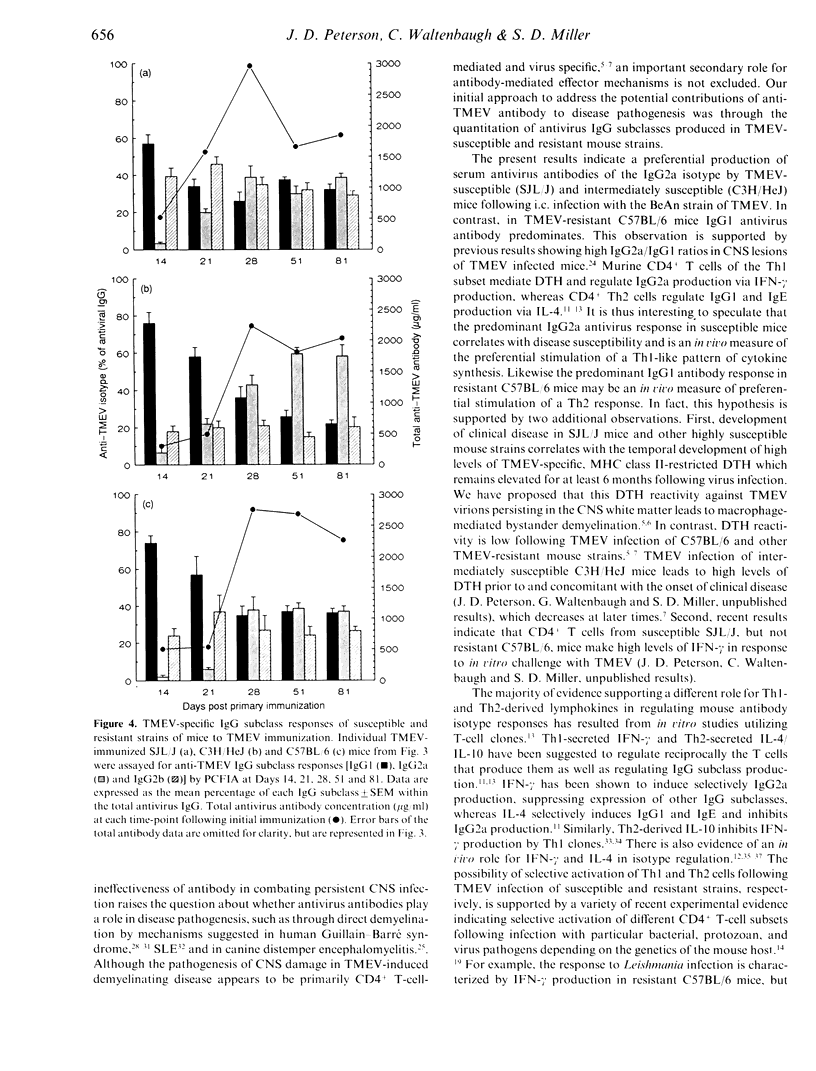
Inbred mouse strains differ in susceptibility to Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. A strong correlation between disease susceptibility and delayed-type hypersensitivity (DTH) has been previously demonstrated, but no strong correlation between disease susceptibility and total anti-TMEV ELISA titres was shown. Since both DTH and IgG2a antibody production are regulated by CD4+ Th1 cells, we investigated three strains of mice to determine whether antivirus IgG2a antibody levels, like DTH in previous studies, correlated with disease susceptibility. Susceptible SJL/J, intermediately susceptible C3H/HeJ, and resistant C57BL/6 mice were infected intracerebrally (i.c.) with the BeAn strain of TMEV and monitored for clinical signs of demyelination and for levels of TMEV-specific antibody of different IgG subclasses using a particle concentration fluorescence immunoassay (PCFIA). Resistant C57BL/6 mice were found to have significantly lower concentrations of total anti-TMEV antibody than susceptible SJL/J mice and intermediately susceptible C3H/HeJ mice show variable antibody responses. A predominance of anti-TMEV IgG2a (Th1 regulated) antibody was seen in susceptible and intermediately susceptible mice, whereas resistant mice displayed a predominant anti-TMEV IgG1 (Th2 regulated) response accompanied by a marked deficiency of IgG2a. In contrast, immunization of C57BL/6 mice with UV-inactivated TMEV in adjuvant revealed that this strain was not defective either in its ability to generate high levels of anti-TMEV antibody or in its ability to produce IgG2a antibody. These results suggest that the antivirus IgG subclass profile is dependent upon the immunization route, virus viability and/or the use of adjuvant and that the levels of antivirus subclasses may be predictive of disease susceptibility.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass H., Mosmann T., Strober S. Evidence for mouse Th1- and Th2-like helper T cells in vivo. Selective reduction of Th1-like cells after total lymphoid irradiation. J Exp Med. 1989 Nov 1;170(5):1495–1511. doi: 10.1084/jem.170.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carding S. R., West J., Woods A., Bottomly K. Differential activation of cytokine genes in normal CD4-bearing T cells is stimulus dependent. Eur J Immunol. 1989 Feb;19(2):231–238. doi: 10.1002/eji.1830190203. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Lipton H. L., Miller S. D. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986 Feb 1;136(3):920–927. [PubMed] [Google Scholar]
- Clatch R. J., Lipton H. L., Miller S. D. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. II. Survey of host immune responses and central nervous system virus titers in inbred mouse strains. Microb Pathog. 1987 Nov;3(5):327–337. doi: 10.1016/0882-4010(87)90003-9. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Melvold R. W., Miller S. D., Lipton H. L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J Immunol. 1985 Aug;135(2):1408–1414. [PubMed] [Google Scholar]
- Cook S. D., Dowling P. C., Murray M. R., Whitaker J. N. Circulating demyelinating factors in acute idiopathic polyneuropathy. Arch Neurol. 1971 Feb;24(2):136–144. doi: 10.1001/archneur.1971.00480320064006. [DOI] [PubMed] [Google Scholar]
- Cook S. D., Dowling P. C. the role of autoantibody and immune complexes in the pathogenesis of Guillain-Barré syndrome. Ann Neurol. 1981;9 (Suppl):70–79. doi: 10.1002/ana.410090712. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987 Jan 1;165(1):64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- Dörries R., Schwender S., Wege H., Harms H., Watanabe R., ter Meulen V. Coronavirus-JHM-induced demyelinating encephalomyelitis in rats. Analysis of the intrathecal immune response. Ann N Y Acad Sci. 1988;540:663–664. doi: 10.1111/j.1749-6632.1988.tb27205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas A. I., Medgyesi G. A., Füst G., Miklós K., Gergely J. Immunogenicity of antigen complexed with antibody. I. Role of different isotypes. Immunology. 1982 Mar;45(3):483–492. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety S. J., Clatch R. J., Lipton H. L., Goswami R. G., Rundell M. K., Miller S. D. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. IV. Identification of an immunodominant T cell determinant on the N-terminal end of the VP2 capsid protein in susceptible SJL/J mice. J Immunol. 1991 Apr 1;146(7):2401–2408. [PubMed] [Google Scholar]
- Griot C., Bürge T., Vandevelde M., Peterhans E. Antibody-induced generation of reactive oxygen radicals by brain macrophages in canine distemper encephalitis: a mechanism for bystander demyelination. Acta Neuropathol. 1989;78(4):396–403. doi: 10.1007/BF00688176. [DOI] [PubMed] [Google Scholar]
- Hauser C., Snapper C. M., Ohara J., Paul W. E., Katz S. I. T helper cells grown with hapten-modified cultured Langerhans' cells produce interleukin 4 and stimulate IgE production by B cells. Eur J Immunol. 1989 Feb;19(2):245–251. doi: 10.1002/eji.1830190205. [DOI] [PubMed] [Google Scholar]
- Hayglass K. T., Gieni R. S., Stefura W. P. Long-lived reciprocal regulation of antigen-specific IgE and IgG2a responses in mice treated with glutaraldehyde-polymerized ovalbumin. Immunology. 1991 Aug;73(4):407–414. [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke J. F., Hyllested K. Multiple sclerosis in the Faroe Islands. II. Clinical update, transmission, and the nature of MS. Neurology. 1986 Mar;36(3):307–328. doi: 10.1212/wnl.36.3.307. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Garrett R. S., Oldstone M. B. Immune complex deposits in the choroid plexus. Birth Defects Orig Artic Ser. 1978;14(5):237–244. [PubMed] [Google Scholar]
- Lehrich J. R., Arnason B. G., Hochberg F. H. Demyelinative myelopathy in mice induced by the DA virus. J Neurol Sci. 1976 Oct;29(2-4):149–160. doi: 10.1016/0022-510x(76)90167-2. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Friedmann A. Purification of Theiler's murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J Virol. 1980 Mar;33(3):1165–1172. doi: 10.1128/jvi.33.3.1165-1172.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magilavy D. B., Fitch F. W., Gajewski T. F. Murine hepatic accessory cells support the proliferation of Th1 but not Th2 helper T lymphocyte clones. J Exp Med. 1989 Sep 1;170(3):985–990. doi: 10.1084/jem.170.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Murray J. S., Madri J., Tite J., Carding S. R., Bottomly K. MHC control of CD4+ T cell subset activation. J Exp Med. 1989 Dec 1;170(6):2135–2140. doi: 10.1084/jem.170.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Pedrazzini T., Farrell J. P., Louis J. T-cell responses and immunity to experimental infection with leishmania major. Annu Rev Immunol. 1989;7:561–578. doi: 10.1146/annurev.iy.07.040189.003021. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Miller A. Epidemiology of multiple sclerosis: critique of the evidence for a viral etiology. Am J Epidemiol. 1978 Jun;107(6):451–461. doi: 10.1093/oxfordjournals.aje.a112564. [DOI] [PubMed] [Google Scholar]
- Parsons L. M., Webb H. E. Identification of immunoglobulin-containing cells in the central nervous system of the mouse following infection with the demyelinating strain of Semliki Forest virus. Br J Exp Pathol. 1989 Jun;70(3):247–255. [PMC free article] [PubMed] [Google Scholar]
- Peterson J. D., Miller S. D., Waltenbaugh C. Rapid biotin-avidin method for quantitation of antiviral antibody isotypes. J Virol Methods. 1990 Feb;27(2):189–201. doi: 10.1016/0166-0934(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Evidence for differential induction of helper T cell subsets during Trichinella spiralis infection. J Immunol. 1989 Dec 15;143(12):4232–4237. [PubMed] [Google Scholar]
- Rodriguez M., Kenny J. J., Thiemann R. L., Woloschak G. E. Theiler's virus-induced demyelination in mice immunosuppressed with anti-IgM and in mice expressing the xid gene. Microb Pathog. 1990 Jan;8(1):23–35. doi: 10.1016/0882-4010(90)90005-b. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Siegel L. M., Hovanec-Burns D., Bologa L., Graves M. C. Theiler's virus-associated antigens on the surfaces of cultured glial cells. Virology. 1988 Oct;166(2):463–474. doi: 10.1016/0042-6822(88)90517-x. [DOI] [PubMed] [Google Scholar]
- Rossi C. P., Cash E., Aubert C., Coutinho A. Role of the humoral immune response in resistance to Theiler's virus infection. J Virol. 1991 Jul;65(7):3895–3899. doi: 10.1128/jvi.65.7.3895-3899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Kaufmann S. H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991 Oct;12(10):346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Foellmer H. G., Madri J. A., Janeway C. A., Jr Inverse Ir gene control of the antibody and T cell proliferative responses to human basement membrane collagen. J Immunol. 1987 Nov 1;139(9):2892–2898. [PubMed] [Google Scholar]
- Vandvik B., Vartdal F., Norrby E. Herpes simplex virus encephalitis: intrathecal synthesis of oligoclonal virus-specific IgG, IgA and IgM antibodies. J Neurol. 1982;228(1):25–38. doi: 10.1007/BF00313407. [DOI] [PubMed] [Google Scholar]


