Abstract
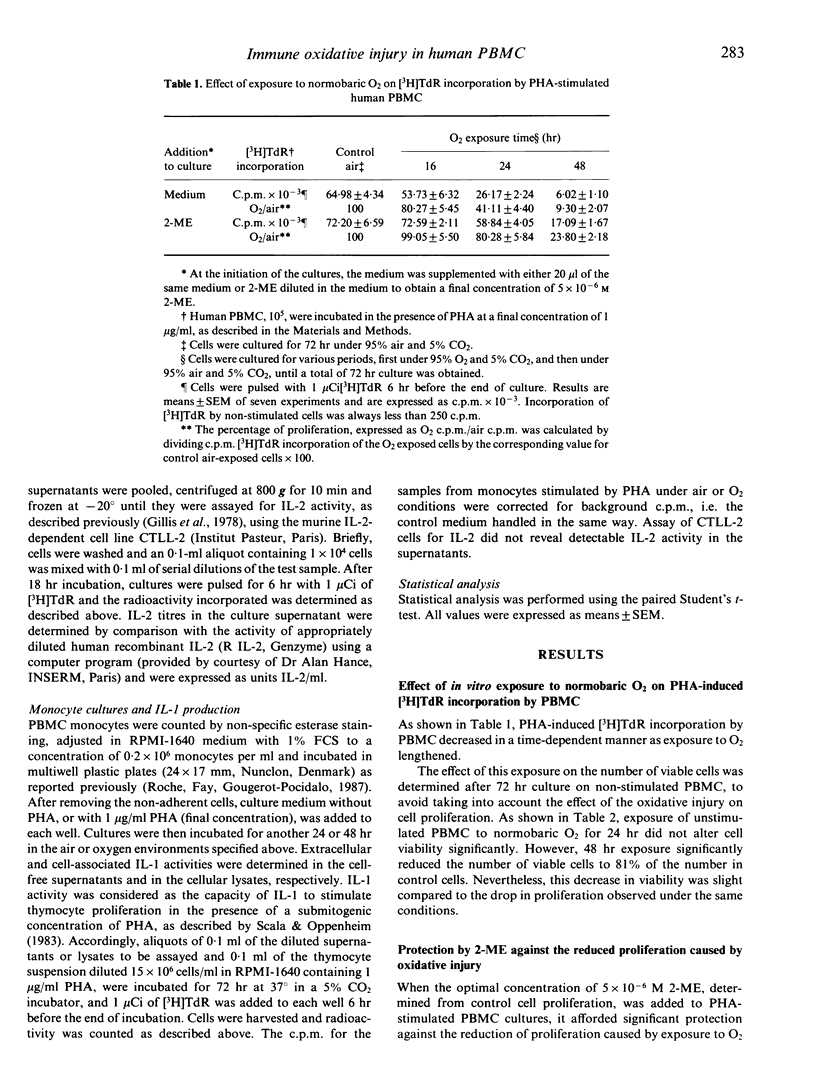
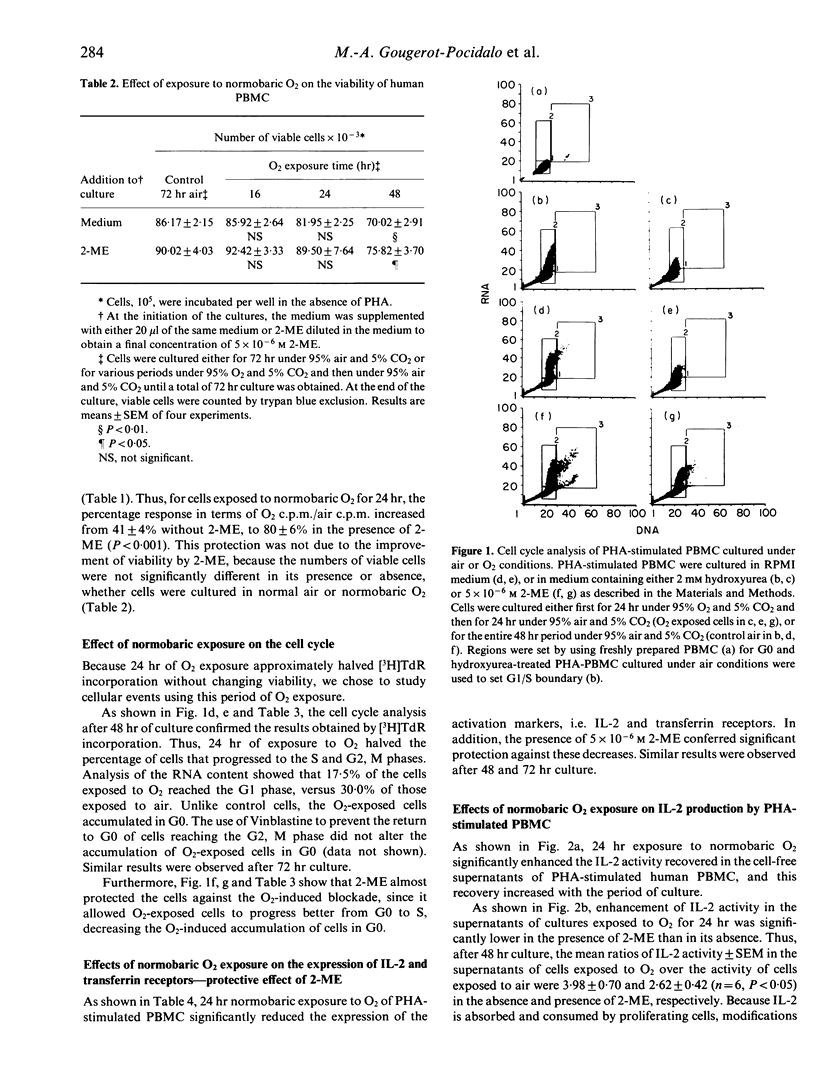
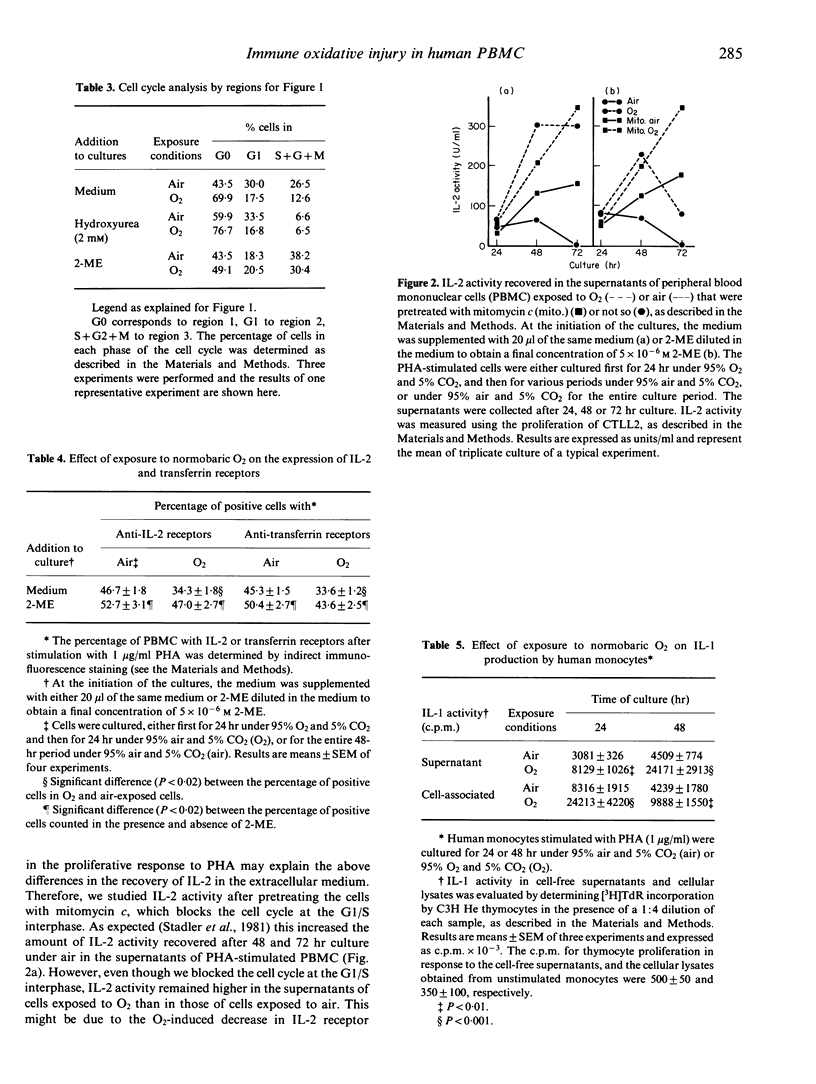
The use of normobaric exposure to O2 as a model for in vitro oxidative injury prevented phytohaemagglutinin (PHA)-stimulated human peripheral blood mononuclear cells (PBMC) from undergoing the G0 to G1 transition, but 5 x 10(-6) M 2-mercaptoethanol (2-ME) almost protected the cells from this blockade. The percentage of cells with IL-2 and transferrin-receptors was reduced by the O2 exposure and, like the cell cycle transition, was protected by 2-ME against oxidative injury. By contrast, IL-2 recovery in the supernatants of O2-exposed PHA-stimulated PBMC was enhanced. This enhancement may be due partly to the reduced IL-2 consumption caused by the decreases in IL-2 receptor expression and in proliferation. On the other hand, IL-2 recovery in the supernatants of O2-treated PBMC was always enhanced compared to the IL-2 control recovery after DNA synthesis was blocked in G1/S by mitomycin c, and the G0/G1 transition was protected by 2-ME. Furthermore, PHA-stimulated monocytes exposed to O2 produced more IL-1 than control cells. This enhanced IL-1 production was not modified by 2-ME. These results suggest that oxidative injury reduces the proliferation of PBMC by interfering with the cellular events that lead to the transition from the G0 to the G1 phase of the cell cycle. The protective effects of 2-ME suggest that thiol compounds have a critical role in the early events of the cell cycle. By contrast, exposure to O2 induced increases in the production of both IL-1 and IL-2 that may not be related to alterations in the thiol status of the cell.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettens F., Kristensen F., Walker C., de Weck A. L. Human lymphocyte proliferation. II. Formation of activated (G1) cells. Eur J Immunol. 1982 Nov;12(11):948–952. doi: 10.1002/eji.1830121110. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chaplin D. D., Wedner J. H. Inhibition of lectin-induced lymphocyte activation by diamide and other sulfhydryl reagents. Cell Immunol. 1978 Mar 15;36(2):303–311. doi: 10.1016/0008-8749(78)90274-5. [DOI] [PubMed] [Google Scholar]
- Chollet-Martin S., Gougerot-Pocidalo M. A. Enumeration of the T4 positive and T8 positive lymphocytes in AIDS and related syndromes. Comparison of the immunogold and the immunofluorescence techniques. J Immunol Methods. 1986 Jul 24;91(2):271–274. doi: 10.1016/0022-1759(86)90489-8. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D., Staiano-Coico L., Sharpless T., Melamed M. R. Relationship between RNA content and progression of lymphocytes through S phase of cell cycle. Proc Natl Acad Sci U S A. 1979 Jan;76(1):358–362. doi: 10.1073/pnas.76.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria G., Agarossi G., Adorini L. Selective effects of ionizing radiations on immunoregulatory cells. Immunol Rev. 1982;65:23–54. doi: 10.1111/j.1600-065x.1982.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Fischman C. M., Udey M. C., Kurtz M., Wedner H. J. Inhibition of lectin-induced lymphocyte activation by 2-cyclohexene-1-one: decreased intracellular glutathione inhibits an early event in the activation sequence. J Immunol. 1981 Dec;127(6):2257–2262. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gougerot-Pocidalo M. A., Fay M., Pocidalo J. J. In vivo normobaric oxygen exposure depresses spleen cell in vitro Con A response. Effects of 2-mercaptoethanol and peritoneal cells. Clin Exp Immunol. 1984 Nov;58(2):428–435. [PMC free article] [PubMed] [Google Scholar]
- Gougerot-Pocidalo M. A., Fay M., Roche Y., Lacombe P., Marquetty C. Immune oxidative injury induced in mice exposed to normobaric O2: effects of thiol compounds on the splenic cell sulfhydryl content and Con A proliferative response. J Immunol. 1985 Sep;135(3):2045–2051. [PubMed] [Google Scholar]
- Harman D., Heidrick M. L., Eddy D. E. Free radical theory of aging: effect of free-radical-reaction inhibitors on the immune response. J Am Geriatr Soc. 1977 Sep;25(9):400–407. doi: 10.1111/j.1532-5415.1977.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg I., Lindahl-Kiessling K., Löw H., Mattsson A. Role of aerobic conditions in the control of cell proliferation. Int Arch Allergy Appl Immunol. 1981;65(3):250–256. doi: 10.1159/000232764. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- Kraus L., Gougerot-Pocidalo M. A., Lacombe P., Pocidalo J. J. Depression of Con A proliferative response of immune cells by in vitro hyperoxic exposure--protective effects of thiol compounds. Int J Immunopharmacol. 1985;7(5):753–760. doi: 10.1016/0192-0561(85)90162-6. [DOI] [PubMed] [Google Scholar]
- Levacher-Place M., Gougerot-Pocidalo M. A., Rouveix B., Kraus L., Pocidalo J. J. Characterization of immunological depression in mice exposed to normobaric oxygen. Clin Exp Immunol. 1983 Nov;54(2):580–586. [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J., Lawrence D. A. Determination of glutathione in lymphocytes and possible association of redox state and proliferative capacity of lymphocytes. Biochem J. 1981 Sep 15;198(3):571–579. doi: 10.1042/bj1980571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yamamoto I. Mechanism of augmentation of the antibody response in vitro by 2-mercaptoethanol in murine lymphocytes. I. 2-Mercaptoethanol-induced stimulation of the uptake of cystine, an essential amino acid. J Exp Med. 1982 May 1;155(5):1277–1290. doi: 10.1084/jem.155.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yamamoto I. Mechanism of augmentation of the antibody response in vitro by 2-mercaptoethanol in murine lymphocytes. II. A major role of the mixed disulfide between 2-mercaptoethanol and cysteine. Cell Immunol. 1983 Jul 1;79(1):173–185. doi: 10.1016/0008-8749(83)90060-6. [DOI] [PubMed] [Google Scholar]
- Roche Y., Fay M., Gougerot-Pocidalo M. A. Effects of quinolones on interleukin 1 production in vitro by human monocytes. Immunopharmacology. 1987 Apr;13(2):99–109. doi: 10.1016/0162-3109(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Roth S., Dröge W. Regulation of T-cell activation and T-cell growth factor (TCGF) production by hydrogen peroxide. Cell Immunol. 1987 Sep;108(2):417–424. doi: 10.1016/0008-8749(87)90224-3. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Kamps S., Campbell R. The effect of oxidant injury on the lymphoblastic transformation of human lymphocytes. Photochem Photobiol. 1978 Oct-Nov;28(4-5):909–915. doi: 10.1111/j.1751-1097.1978.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler B. M., Dougherty S. F., Farrar J. J., Oppenheim J. J. Relationship of cell cycle to recovery of IL 2 activity from human mononuclear cells, human and mouse T cell lines. J Immunol. 1981 Nov;127(5):1936–1940. [PubMed] [Google Scholar]
- Trush M. A., Mimnaugh E. G., Gram T. E. Activation of pharmacologic agents to radical intermediates. Implications for the role of free radicals in drug action and toxicity. Biochem Pharmacol. 1982 Nov 1;31(21):3335–3346. doi: 10.1016/0006-2952(82)90609-8. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Ransil B. J., Shapiro H. M., Strom T. B. Accessory cell requirement for activation antigen expression and cell cycle progression by human T lymphocytes. J Immunol. 1984 Dec;133(6):2986–2995. [PubMed] [Google Scholar]
- Zmuda J., Friedenson B. Changes in intracellular glutathione levels in stimulated and unstimulated lymphocytes in the presence of 2-mercaptoethanol or cysteine. J Immunol. 1983 Jan;130(1):362–364. [PubMed] [Google Scholar]


