Abstract
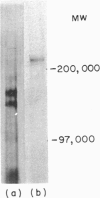
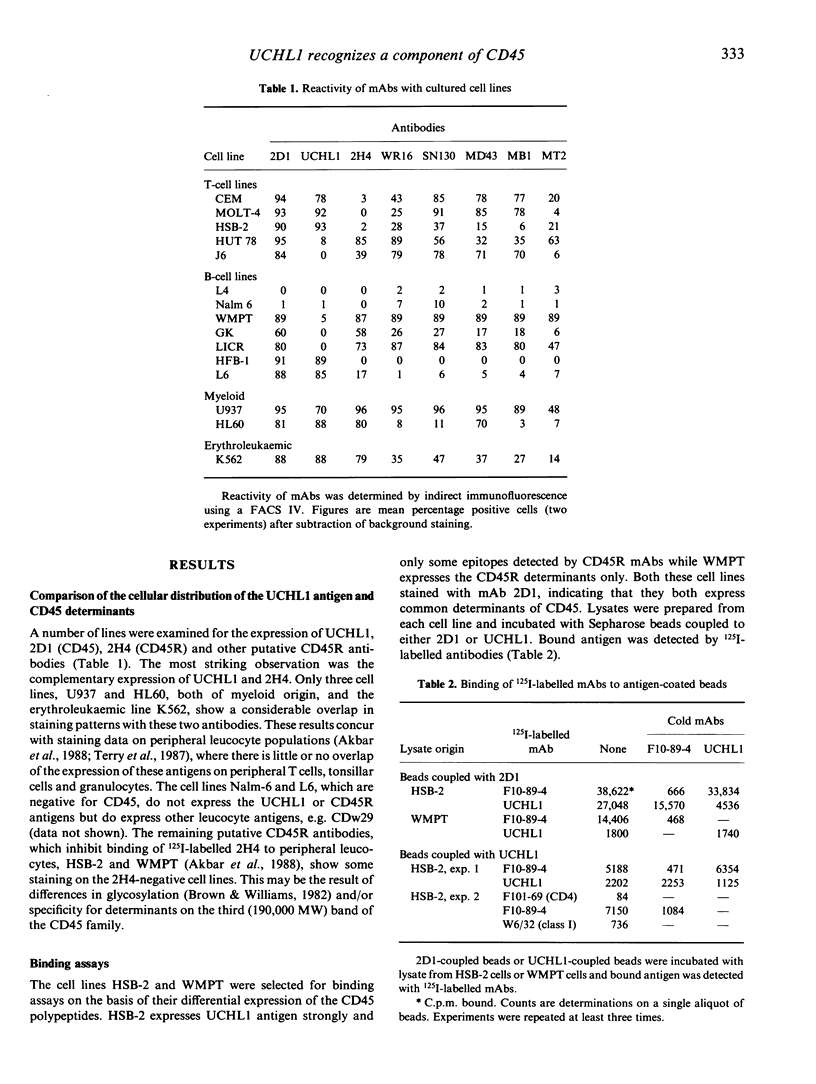
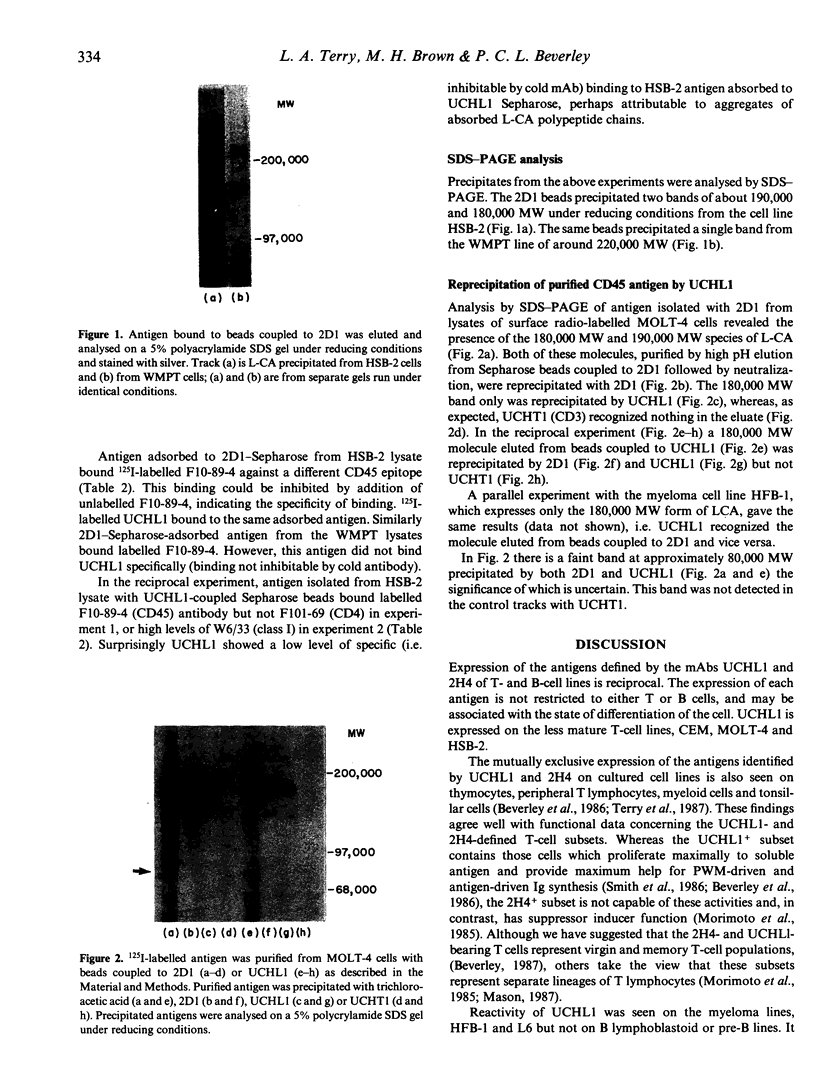
The leucocyte-common antigen (L-CA or CD45) is a family of high molecular weight glycoproteins, ranging from 180,000 to 220,000 MW that are expressed only on cells of lymphoid and myeloid origin. CD45 monoclonal antibodies (mAbs) recognize epitopes present on all polypeptides of the family, while other mAbs, termed CD45R, recognize determinants found only on the 220,000 MW and 200,000 MW polypeptides. In contrast the mAb UCHL1 recognizes a 180,000 MW antigen. UCHL1-coupled Sepharose beads were used to absorb antigen from lysates of cell lines. CD45 mAbs bound to this immobilized antigen. Antigen immobilized with CD45 mAb-coupled Sepharose beads bound UCHL1. Antigen purified by absorption and elution from the MOLT-4 cell line with CD45 mAb-coupled beads yielded molecules of 180,000 and 190,000 MW. Reprecipitation of the eluted antigen with UCHL1 resulted in a 180,000 MW band only. In a reciprocal experiment, CD45 mAb reprecipitated a 180,000 MW molecule from purified UCHL1 antigen. UCHL1 and the CD45R mAb 2H4 showed a mutually exclusive pattern of reactivity with human T- and B-cell lines, but co-expression of the antigens was seen on two myeloid and one erythroleukaemic cell line. In contrast, epitopes recognized by other putative CD45R mAbs were co-expressed with UCHL1 both on myeloid, erythroid and many T- and B-cell lines. We conclude that UCHL1 recognizes an epitope present only on the 180,000 MW polypeptide of CD45. Expression of this antigen is essentially reciprocal to the epitope detected by the CD45R mAb 2H4.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C. Human T cell subsets. Immunol Lett. 1987 Apr;14(4):263–267. doi: 10.1016/0165-2478(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Participation of histocompatibility antigens in capping of molecularly independent cell surface components by their specific antibodies. Proc Natl Acad Sci U S A. 1978 May;75(5):2406–2410. doi: 10.1073/pnas.75.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Suchard S. J., Nagpal M. L., Glenney J. R., Jr A T-lymphoma transmembrane glycoprotein (gp180) is linked to the cytoskeletal protein, fodrin. J Cell Biol. 1985 Aug;101(2):477–487. doi: 10.1083/jcb.101.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradstock K. F., Janossy G., Pizzolo G., Hoffbrand A. V., McMichael A., Pilch J. R., Milstein C., Beverley P., Bollum F. J. Subpopulations of normal and leukemic human thymocytes: an analysis with the use of monoclonal antibodies. J Natl Cancer Inst. 1980 Jul;65(1):33–42. [PubMed] [Google Scholar]
- Brown W. R., Williams A. F. Lymphocyte cell surface glycoproteins which bind to soybean and peanut lectins. Immunology. 1982 Aug;46(4):713–726. [PMC free article] [PubMed] [Google Scholar]
- Burns G. F., Werkmeister J. A., Triglia T. A novel antigenic cell surface protein associated with T200 is involved in the post-activation stage of human NK cell-mediated lysis. J Immunol. 1984 Sep;133(3):1391–1396. [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification with a monoclonal antibody of a predominantly B lymphocyte-specific determinant of the human leukocyte common antigen. Evidence for structural and possible functional diversity of the human leukocyte common molecule. J Exp Med. 1981 Apr 1;153(4):753–765. doi: 10.1084/jem.153.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Fabre J. W., Williams A. F. Quantitative serological analysis of a rabbit anti-rat lymphocyte serum and preliminary biochemical characterisation of the major antigen recognised. Transplantation. 1977 Apr;23(4):349–359. doi: 10.1097/00007890-197704000-00009. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp J. A., Davis B. S., Ewald S. J. Inhibition of T cell responses to alloantigens and polyclonal mitogens by Ly-5 antisera. J Immunol. 1984 Jul;133(1):10–15. [PubMed] [Google Scholar]
- Koning F., Bakker A., Dubelaar M., Lesslauer W., Mullink R., Schuits R., Ligthart G., Naipal A., Bruning H. Identification of a new lymphocyte subset surface antigen, the expression of which disappears after in vitro and in vivo stimulation. Distribution, biochemistry, and functional studies. Hum Immunol. 1986 Nov;17(3):325–342. doi: 10.1016/0198-8859(86)90284-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Lefrancois L., Puddington L., Machamer C. E., Bevan M. J. Acquisition of cytotoxic T lymphocyte-specific carbohydrate differentiation antigens. J Exp Med. 1985 Oct 1;162(4):1275–1293. doi: 10.1084/jem.162.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W. Subpopulations of T lymphocytes. Immunol Lett. 1987 Apr;14(4):269–270. doi: 10.1016/0165-2478(87)90002-2. [DOI] [PubMed] [Google Scholar]
- Moore K., Nesbitt A. M. Identification and isolation of OKT4+ suppressor cells with the monoclonal antibody WR16. Immunology. 1986 Aug;58(4):659–664. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nieminen P., Saksela E. NK-9, a distinct sialylated antigen of the T200 family. Eur J Immunol. 1986 May;16(5):513–518. doi: 10.1002/eji.1830160509. [DOI] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow R. L., McKenzie I. F. A function for human T200 in natural killer cytolysis. Transplantation. 1983 Aug;36(2):166–171. doi: 10.1097/00007890-198308000-00011. [DOI] [PubMed] [Google Scholar]
- Standring R., McMaster W. R., Sunderland C. A., Williams A. F. The predominant heavily glycosylated glycoproteins at the surface of rat lymphoid cells are differentiation antigens. Eur J Immunol. 1978 Dec;8(12):832–839. doi: 10.1002/eji.1830081203. [DOI] [PubMed] [Google Scholar]
- Starling G. C., Davidson S. E., McKenzie J. L., Hart D. N. Inhibition of natural killer-cell mediated cytolysis with monoclonal antibodies to restricted and non-restricted epitopes of the leucocyte common antigen. Immunology. 1987 Jul;61(3):351–356. [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Mazauskas C. Immunological properties of murine thymus-dependent lymphocyte surface glycoproteins. Eur J Immunol. 1976 Aug;6(8):557–562. doi: 10.1002/eji.1830060806. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]