Abstract
A small proportion of breast cancers are due to a heritable predisposition. Recently, two predisposition genes, BRCA1 and BRCA2, have been identified and cloned. The morphological features of tumours from patients harbouring mutations in the BRCA1 and BRCA2 genes differ from each other and from sporadic breast cancers. Both are of higher grade than are sporadic cases. An excess of medullary/atypical medullary carcinoma has been reported in patients with BRCA1 mutations. Multifactorial analysis, however, shows that the only features independently associated with BRCA1 mutations are a high mitotic count, pushing tumour margins and a lymphocytic infiltrate. For BRCA2 mutation, an association with tubular/lobular carcinoma has been suggested, but not substantiated in a larger Breast Cancer Linkage Consortium study. In multifactorial analysis, the independent features were a lack of tubule formation and pushing tumour margins only. The morphological analysis has implications for clinical management of patients.
Keywords: BRCA1, BRCA2, familial, pathology
Introduction
Within the developed countries breast cancer is the commonest malignancy in women. It is estimated that approximately one in 12 women will develop breast cancer in their lifetime.
The majority of breast cancers (95%) are sporadic; only a small proportion, particularly those diagnosed in young women, are due to a highly penetrant autosomal-dominant trait. Over the past 5 years there has been considerable progress in the identification and localization of the genes responsible for hereditary breast cancer. Two in particular have grabbed the headlines; these are BRCA1 and BRCA2 [1,2].
For more than 50 years, researchers have been fascinated by the association of histopathological cancer type with a positive family history of breast cancer. Certain types, including medullary carcinoma, tubular carcinoma, lobular carcinoma in situ and invasive lobular carcinoma (Fig. 1a1b1c1d), have been reported [3,4,5,6,7] to be found more commonly in association with a positive family history of breast cancer than have other subtypes. Some of the reported studies have been difficult to interpret because of the small number of samples, the differing criteria for a positive family history and the controversies surrounding the classification of breast cancer. The histopathological classification of breast disease is subjective and, despite an attempt to provide clear guidelines, the interobserver variability is known to be high [8**]. Because of the subjective nature of histological examination and the factors outlined above, no clear agreement has emerged that any particular phenotype is more commonly associated with a positive family history than any other. Nonetheless, in a histological review of the population-based series of 4071 breast cancers diagnosed in women between the ages of 20 and 54 years in the Cancer and Steroids Hormone study [9], lobular carcinoma in situ showed a strong association with familial risk. In the Utah population database [10], invasive lobular carcinoma has been shown to have an association with familiality. Since the localization and identification of breast cancer predisposition genes BRCA1 and BRCA2, pathologists have tried to identify the morphological phenotypes for these two genes.
Figure 1.
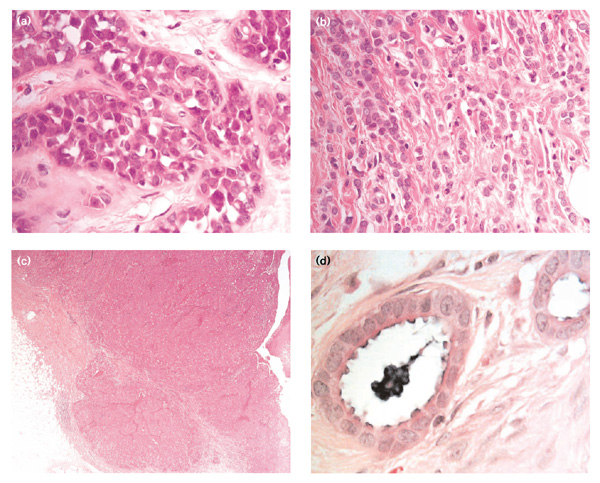
Histopathological cancer types reported to be associated with a positive family history of breast cancer. (a) Lobular carcinoma in situ.(b) Invasive lobular carcinoma. (c) Medullary carcinoma. (d) Tubular carcinoma.
Histopathological features of BRCA1-associated tumours
There are a number of reports in the literature [11,12,13**,14*] that indicate that breast cancers arising in patients with BRCA1 mutations are of higher grade than are sporadic cancers. Eisenger et al [14] studied 27 BRCA1-associated tumours from 14 families and compared these with sporadic breast carcinomas, matching for grade. They found an excess of grade III carcinomas in BRCA1-associated tumours. Marcus et al [13**] reported the first large series of the pathology of BRCA1-related tumours. In their study, they assigned 90 breast cancer patients to the BRCA1 group on the basis of linkage to chromosomes 17q and/or the presence of ovarian and male breast cancer. The control set comprised 187 predominantly nonfamilial breast cancer patients. The tumours were analyzed for histological type, grade, ploidy and S-phase fraction. The investigators found that BRCA1-associated tumours were more likely to be of medullary or atypical medullary type, and to be of higher grade, were more frequently aneuploid and had higher tumour cell proliferation rates. The medullary association lost formal significance when adjusted for age.
Recently the pathology of breast cancers related to BRCA1 mutations were examined in a very large, collaborative study organized through the Breast Cancer Linkage Consortium [15**,16**], and the histological findings were compared with those in control individuals who did not have a family history of the disease. There were 118 (27%) patients who were assigned to the BRCA1 group on the basis of linkage or mutational data. The control group comprised 548 breast cancer patients who did not have a known family history. Seven pathologists, all of whom had experience of breast pathology, carried out the review. The pathologists were unaware as to whether the slides were from the familial or sporadic cases. The cancers were typed and graded using the criteria used by the UK National Breast Screening Programme [17] as follows. They were graded by giving a score of 1-3 for each parameter. If more than 75% of the tumour has good tubules, the score is 1; if less than 10% of the tumour has good tubules, the score is 3. For pleomorphism, the greater the degree of pleomorphism, the worse the score. Similarly, the higher the mitotic count per 10 high-power fields, the higher the score. Total scores of 3-5, 6-7 and 8-9 mean that the tumour is of grades I, II and III, respectively.
The results from this large review of histopathological material [15**,16**] produced some intriguing findings. No differences were found, between BRCA1 and control breast cancers, in the proportion of the invasive ductal carcinoma of no special type. In keeping with the study by Marcus et al [13**], more carcinomas were recorded as medullary or atypical medullary in the BRCA1 group (14%) than in the control group (2%; P <0.0001). The overall grade for BRCA1 breast cancers was significantly higher than that in the control population breast cancers [15**,16**]. Interestingly the higher grade of the BRCA1 tumours was a result of higher score of all three parameters of grade (tubule formation, pleomorphism and mitosis).
The presence of in-situ disease was also recorded from the analysis. The results do not represent an accurate assessment of the presence or extent of in-situ disease, because the breasts of neither the control nor familial patients were examined extensively. Nevertheless the sampling problem for the in-situ disease was the same for both familial and control patients.
Ductal carcinoma in situ was seen less frequently in BRCA1 cases (41%) than in control individuals (56%; P = 0.01). Lobular carcinoma in situ was also seen less frequently in control individuals, but the results were not statistically significant.
Because of the strong associations of the medullary and atypical medullary carcinoma with the BRCA1 phenotype, a further review to identify the features that were predictive for BRCA1 phenotype was carried out [16**]. Medullary carcinoma is a controversial entity. It is defined as a tumour that grows in solid sheets within an indistinct cell border (syncytial growth pattern), has large vesicular nuclei and prominent nucleoli, a broad pushing margin and a prominent lymphocytic infiltrate both at the periphery and within the tumour. These features must be present in the entire tumour for it to be regarded as a classical medullary carcinoma [17,18*]. If the tumour has less lymphocytic infiltrate or an infiltrating margin in part of the tumour, it is regarded as an atypical medullary carcinoma. The presence of a classical ductal carcinoma of no special type forming less than 25% of the tumour also pushes it into an atypical medullary carcinoma category. Although these features appear to be fairly specific, pathologists have a great deal of difficulty in making a diagnosis of medullary and atypical medullary carcinoma, and, as in this study, the interobserver agreement is low. Hence, in the second review [16**] the pathologists were asked to evaluate specific features, rather than assigning a specific type to the tumour. They were asked to complete a proforma that included the following: an assessment of the percentage of tumour present as solid sheets of cells (<25%, 25-75%, >75%); the total mitotic count per 10 high-power fields; the presence of continuous pushing margins; the presence of confluent necrosis; the presence of a lymphocytic infiltrate; and, if present, whether mild or prominent, the presence of discernible cell borders; the presence of vesicular nuclei; and the presence of prominent eosinophilic nucleoli.
In a multifactorial analysis using the data from both reviews [15**,16**], the only factors found to be significant were total mitotic count, continuous pushing margins, and lymphocytic infiltrate (Fig. 2 and 3). All other features including the diagnosis of medullary and atypical medullary carcinoma were no longer significant in this multifactorial analysis [16**].
Figure 2.
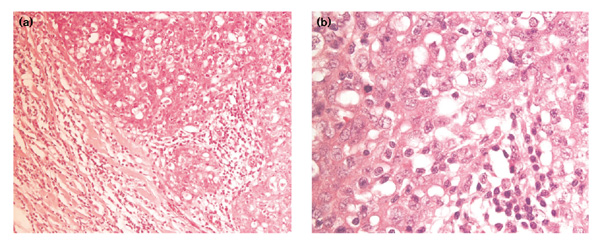
Features reported to be predictive for BRCA1 phenotype. (a) Low power view (×100 magnification) showing the tumour with broad pushing margin. A prominent lymphoid infiltrate is also seen. (b) High-power view (×400 magnification) showing the tumour cells with mitotic activity and the lymphoid infiltrate.
Two of the three features that are independently associated with cancers from the BRCA1 patient (continuous pushing margins and lymphocytic infiltrate) are part of the subset of the characteristics that define medullary carcinoma. High mitotic count, which is the third feature associated with these tumours, is also often seen in medullary carcinomas, because these tend to be of higher grade, but it is not regarded as a defining feature. It appears that, although an increase in the frequency of classical and atypical medullary carcinoma may contribute to the observed BRCA1 phenotype, these cancers probably account for only a small proportion of the differences observed between those with and those without BRCA1 mutation carriers.
Histopathological features of BRCA2-associated tumours
Unlike BRCA1, data on the pathology of tumours associated with BRCA2 are limited. The study by Marcus et al [13**] attempted to delineate the pathology of BRCA2 tumours. Their study groups comprised BRCA1-associated tumours and 'others', which included BRCA2 cases. Although this latter group included 85 patients, only nine were linked to BRCA2, and three were of male breast cancer. The authors suggested that tumours arising in patients with BRCA2 mutations were different from those arising in patients with BRCA1 mutations. These tumours were of lower grade than BRCA1 tumours, were less aneuploid, and did not have the high proliferation seen in tumours from BRCA1 patients. They found an association of BRCA2 tumours with invasive lobular carcinoma, tubular-lobular carcinoma, tubular carcinoma and cribriform carcinoma, which they designated as a 'tubular-lobular group'. This is in contrast to the findings of Agnarsson et al [19*], who found that BRCA2 tumours in the Icelandic population were of higher grade than that in sporadic cases. Their data are, however, based on one particular BRCA2 mutation - 999del5 - and hence it is not possible to rule out that this phenotype represents a peculiarity of this particular mutation.
The studies carried out by the Breast Cancer Linkage Consortium analyzed 78 (18%) patients assigned to the BRCA2 group [15**].This represents the largest set of data on BRCA2 tumours to date. Unlike in the study by Marcus et al [13**], no difference in the frequency of the invasive lobular carcinoma or tubular carcinoma between control individuals and the BRCA2 mutations group was identified. In fact, no BRCA2 mutations carriers had tubular carcinomas, compared with the 5% of the control population. There was also no evidence of an excess of medullary or atypical medullary carcinoma in the BRCA2 group [15**].
BRCA2 breast cancers were overall of higher grade than those from the control population. Interestingly, in contrast to BRCA1, the higher grade of BRCA2 tumour was only due to higher score for tubule formation. No differences were identified in pleomorphism or mitotic count between BRCA2 tumours and sporadic cancers.
There was no difference in the incidence of ductal carcinoma in situ between BRCA2 group and control cancers. Lobular carcinoma in situ was seen less frequently in BRCA2 mutation carriers than in control individuals, but the results were not statistically significant.
Multifactorial analysis from the two reviews performed for the BRCA2 mutation carriers [16**] showed that the only significant features were tubule score and continuous pushing margins.
Conclusion
The studies to date indicate that breast tumours arising in patients with BRCA1 mutations are different from those arising in patients with BRCA2 mutations and from non-familial cancers. The principle differences are in total mitotic count, tubule formation and lymphocytic infiltrate. Patients with BRCA2 mutations also have a high rate of male breast cancer and lower incidence of ovarian cancer.Taken together, the differences in the clinical phenotypes associated with mutations in the two genes suggests that the biological activities of the proteins encoded by the BRCA1 and BRCA2 genes are probably different.
References
- Miki Y, Swensen J, Shattuck E, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. . Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. . Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Erdreich LS, Asal NR, Hoge AF. Morphologic types of breast cancer: age, bilaterality, and family history. South Med J. 1980;73:28–32. doi: 10.1097/00007611-198001000-00012. [DOI] [PubMed] [Google Scholar]
- Lagios MD, Rose MR, Margolin FR. Tubular carcinoma of the breast: association with multicentricity, bilaterality, and family history of mammary carcinoma. Am J Clin Pathol. 1980;73:25–30. doi: 10.1093/ajcp/73.1.25. [DOI] [PubMed] [Google Scholar]
- Rosen PP, Lesser ML, Senie RT, Kinne DW. Epidemiology of breast carcinoma III: relationship of family history to tumor type. . Cancer. 1982;50:171–179. doi: 10.1002/1097-0142(19820701)50:1<171::aid-cncr2820500132>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- LiVolsi VA, Kelsey JL, Fischer DB, et al. Effect of age at first childbirth on risk of developing specific histologic subtype of breast cancer. Cancer. 1982;49:1937–1940. doi: 10.1002/1097-0142(19820501)49:9<1937::aid-cncr2820490931>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Albano WA, Heieck JJ, et al. Genetics, biomarkers, and control of breast cancer: a review. Cancer Genet Cytogenet. 1984;13:43–92. doi: 10.1016/0165-4608(84)90087-6. [DOI] [PubMed] [Google Scholar]
- Sloane JP, Ellman R, Anderson TJ, et al. Consistency of histopatho logical reporting of breast lesions detected by screening: findings of the UK National External Quality Assessment (EQA) Scheme. Eur J Cancer. 1994;30A:1414–1419. doi: 10.1016/0959-8049(94)00261-3. [DOI] [PubMed] [Google Scholar]
- Claus EB, Risch N, Thompson WD, Carter D. Relationship between breast histopathology and family history of breast cancer. Cancer. 1993;71:147–153. doi: 10.1002/1097-0142(19930101)71:1<147::aid-cncr2820710124>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright LA, Thomas A, Goldgar DE, et al. Familiality of cancer in Utah. Cancer Res. 1994;54:2378–2385. [PubMed] [Google Scholar]
- Bignon YJ, Fonck Y, Chassagne MC. Histoprognostic grade in tumours from families with hereditary predisposition to breast cancer [letter]. Lancet. 1995;346:258. doi: 10.1016/s0140-6736(95)91310-6. [DOI] [PubMed] [Google Scholar]
- Jacquemier J, Eisinger F, Birnbaum D, Sobol H. Histoprognostic grade in BRCA1 -associated breast [letter]. . Lancet. 1995;345:1503. doi: 10.1016/s0140-6736(95)91060-3. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Watson P, Page DL, et al. Hereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer. 1996;77:697–709. doi: 10.1002/(SICI)1097-0142(19960215)77:4<697::AID-CNCR16>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Eisinger F, Stoppa-Lyonnet D, Longy M, et al. Germ line mutation at BRCA1 affects the histoprognostic grade in hereditary breast cancer. Cancer Res. 1996;56:471–474. [PubMed] [Google Scholar]
- Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases. Lancet. 1997;349:1505–1510. [PubMed] [Google Scholar]
- Lakhani SR, Jacquemier J, Sloane JP, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90:1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- UK National Coordinating Group for Breast Screening Pathology Pathology Reporting in Breast Cancer Screening. Sheffield: NHSBSB publications. 1995.
- Ridolfi RL, Rosen PP, Port A, Kinne D, Mike V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow up. Cancer. 1977;40:1365–1385. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Agnarsson BA, Jonasson JG, Bjornsdottir IB, et al. Inherited BRCA2 mutation associated with high grade breast cancer. Breast Cancer Res Treat. 1998;47:121–127. doi: 10.1023/a:1005853022804. [DOI] [PubMed] [Google Scholar]


