Abstract
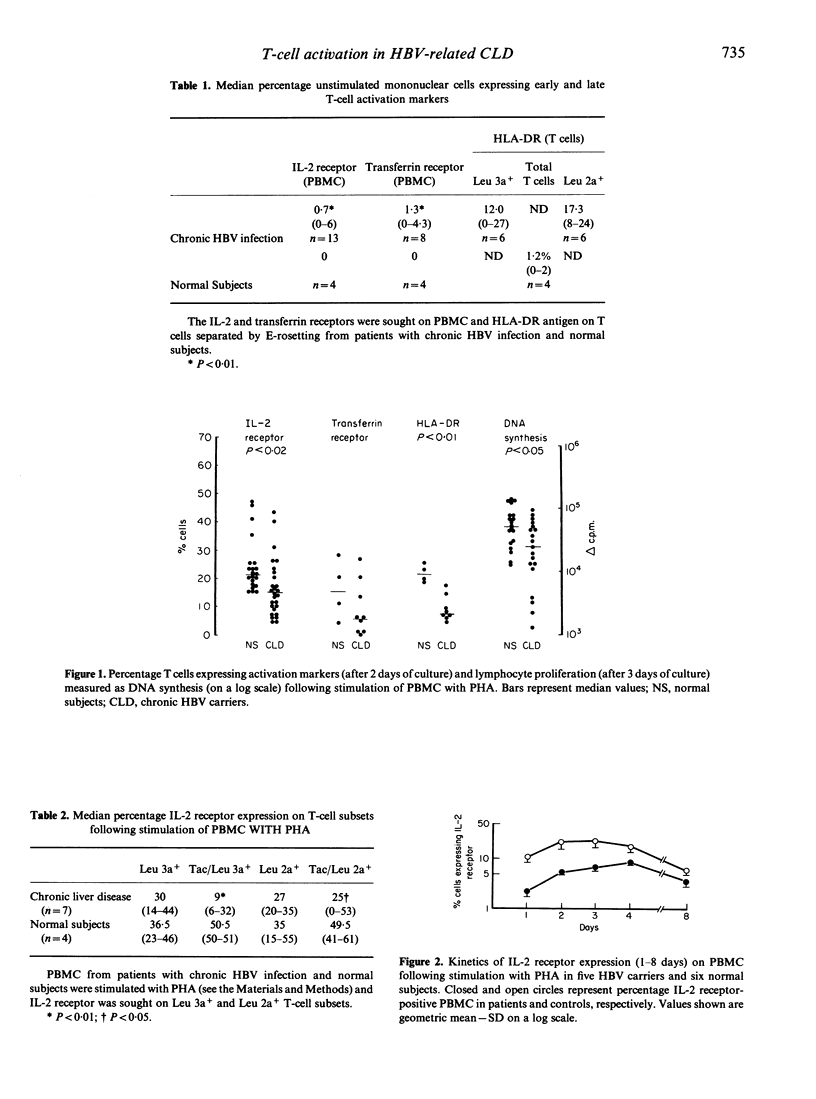
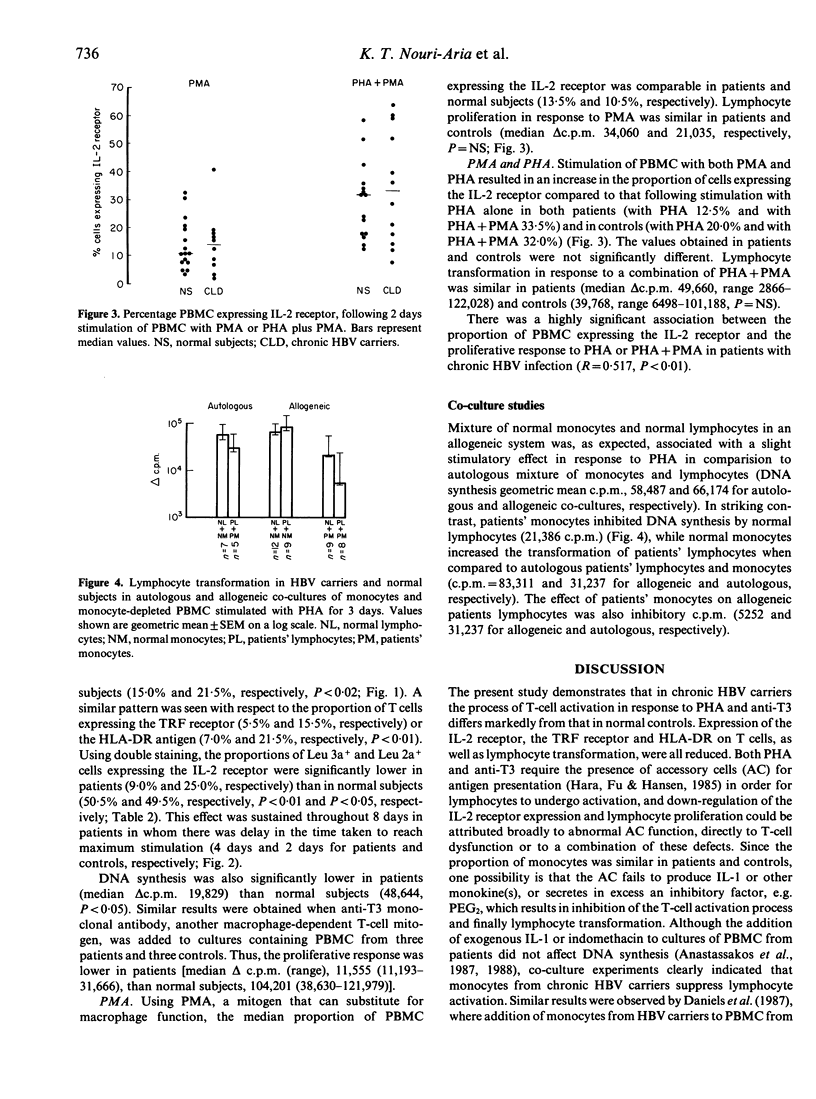
The process of T-cell activation in chronic hepatitis B virus (HBV) carriers has been investigated by measurement of membrane expression of lymphocyte-activation markers in response to a variety of mitogenic stimuli in order to delineate further the abnormality of T-cell-mediated immunity present in such patients. A substantial proportion of unstimulated T cells from the peripheral blood of patients but not controls expressed HLA-DR; in contrast the IL-2 and transferrin receptors were rarely expressed spontaneously in either group and there was no difference in spontaneous lymphocyte transformation. After stimulation with monocyte-dependent T-cell mitogens, phytohaemagglutinin (PHA) or anti-T3, patients had significantly reduced expression of the IL-2 and transferrin receptors and of HLA-DR in association with impaired lymphocyte transformations compared to controls. In contrast, lymphocyte activation was normal in response to the monocyte-independent T-cell mitogen phorbol-myristate-acetate (PMA). These data confirm that the process of T-cell activation is abnormal in chronic HBV carriers but suggest that the T cell is intrinsically normal. In allogeneic co-cultures, monocytes from patients inhibited the transformation of normal and patients' lymphocytes in response to PHA, suggesting that defects of T-cell-mediated immunity in chronic HBV carriers may be a consequence of monocyte dysfunction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastassakos C., Alexander G. J., Wolstencroft R. A., Avery J. A., Portmann B. C., Panayi G. S., Dumonde D. C., Eddleston A. L., Williams R. Interleukin-1 and interleukin-2 activity in chronic hepatitis B virus infection. Gastroenterology. 1988 Apr;94(4):999–1005. doi: 10.1016/0016-5085(88)90559-8. [DOI] [PubMed] [Google Scholar]
- Anastassakos C., Alexander G. J., Wolstencroft R. A., Dumonde D. C., Eddleston A. L., Williams R. Failure of exogenous interleukin 1 and interleukin 2 to correct decreased lymphocyte transformation in chronic hepatitis B virus carriers. Clin Exp Immunol. 1987 Apr;68(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- Barker L. F., Murray R. Aquisition of hepatitis-associated antigen. Clinical features in young adults. JAMA. 1971 Jun 21;216(12):1970–1976. [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Stevens C. E., Lin C. C., Hsieh F. J., Wang K. Y., Sun T. S., Szmuness W. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983 Mar-Apr;3(2):135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- Blumberg B. S., Sutnick A. I., London W. T. Australia antigen as a hepatitis virus. Variation in host response. Am J Med. 1970 Jan;48(1):1–8. doi: 10.1016/0002-9343(70)90093-8. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Castle K. L., Xavier C., Anderson D. S. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. I. Suppressor cell control of T lymphocyte responsiveness. J Immunol. 1981 Jan;126(1):38–44. [PubMed] [Google Scholar]
- Colucci G., Colombo M., Del Ninno E., Paronetto F. In situ characterization by monoclonal antibodies of the mononuclear cell infiltrate in chronic active hepatitis. Gastroenterology. 1983 Nov;85(5):1138–1145. [PubMed] [Google Scholar]
- Davis L., Lipsky P. E. Signals involved in T cell activation. I. Phorbol esters enhance responsiveness but cannot replace intact accessory cells in the induction of mitogen-stimulated T cell proliferation. J Immunol. 1985 Nov;135(5):2946–2952. [PubMed] [Google Scholar]
- Davison F., Alexander G. J., Anastassakos C., Fagan E. A., Williams R. Leucocyte hepatitis B virus DNA in acute and chronic hepatitis B virus infection. J Med Virol. 1987 Aug;22(4):379–385. doi: 10.1002/jmv.1890220411. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Krönke M., Noguchi P. D., Cunningham R. E., Waldmann T. A., Greene W. C. Regulation of interleukin 2 receptor expression: effects of phorbol diester, phospholipase C, and reexposure to lectin or antigen. J Immunol. 1984 Dec;133(6):3054–3061. [PubMed] [Google Scholar]
- Fukui K., Kakumu S., Murakami H., Kuriki J., Yoshioka K., Sakamoto N. Increased peripheral blood Ia positive T cells and their effect on autologous mixed lymphocyte reaction in chronic active liver disease. Clin Exp Immunol. 1984 Oct;58(1):90–96. [PMC free article] [PubMed] [Google Scholar]
- Gerety R. J., Hoofnagle J. H., Markenson J. A., Barker L. F. Exposure to hepatitis B virus and development of the chronic HBAg carrier state in children. J Pediatr. 1974 May;84(5):661–665. doi: 10.1016/s0022-3476(74)80006-5. [DOI] [PubMed] [Google Scholar]
- Giustino V., Dudley F. J., Sherlock S. Thymus-dependent lymphocyte function in patients with hepatitis-associated antigen. Lancet. 1972 Oct 21;2(7782):850–853. doi: 10.1016/s0140-6736(72)92212-x. [DOI] [PubMed] [Google Scholar]
- Hanson R. G., Hoofnagle J. H., Minuk G. Y., Purcell R. H., Gerin J. L. Cell-mediated immunity to hepatitis B surface antigen in man. Clin Exp Immunol. 1984 Aug;57(2):257–264. [PMC free article] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Fu S. M. Human T cell activation. I. Monocyte-independent activation and proliferation induced by anti-T3 monoclonal antibodies in the presence of tumor promoter 12-o-tetradecanoyl phorbol-13 acetate. J Exp Med. 1985 Apr 1;161(4):641–656. doi: 10.1084/jem.161.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nakagawa H., Kobayashi K., Hattori N., Hatano K. Interferon production by peripheral lymphocytes in HBsAg-positive liver diseases. Hepatology. 1982 Nov-Dec;2(6):789–790. doi: 10.1002/hep.1840020607. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Sagnelli E., Manzillo G., Maio G., Pasquale G., Felaco F. M., Filippini P., Izzo C. M., Piccinino F. Serum levels of hepatitis B surface and core antigens during immunosuppressive treatment of HBsAg-positive chronic active hepatitis. Lancet. 1980 Aug 23;2(8191):395–397. doi: 10.1016/s0140-6736(80)90442-0. [DOI] [PubMed] [Google Scholar]
- Saxena S., Nouri-Aria K. T., Anderson M. G., Williams R., Eddleston A. L. In vitro alpha-interferon treatment of peripheral blood mononuclear cells improves interleukin-2 activity in HBV-related chronic liver disease. J Hepatol. 1985;1(4):385–393. doi: 10.1016/s0168-8278(85)80776-5. [DOI] [PubMed] [Google Scholar]
- Stoller A., Collmann R. D. Incidence of infective hepatitis followed by Down's syndrome nine months later. Lancet. 1965 Dec 11;2(7424):1221–1223. doi: 10.1016/s0140-6736(65)90642-2. [DOI] [PubMed] [Google Scholar]
- Viola L., Barrison I. G., Paradinas F., Coleman J. C., Murray-Lyon I. M. Cell-mediated immunity in HBeAg-positive homosexuals with chronic liver disease. Br J Vener Dis. 1982 Feb;58(1):59–61. doi: 10.1136/sti.58.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]


