Abstract
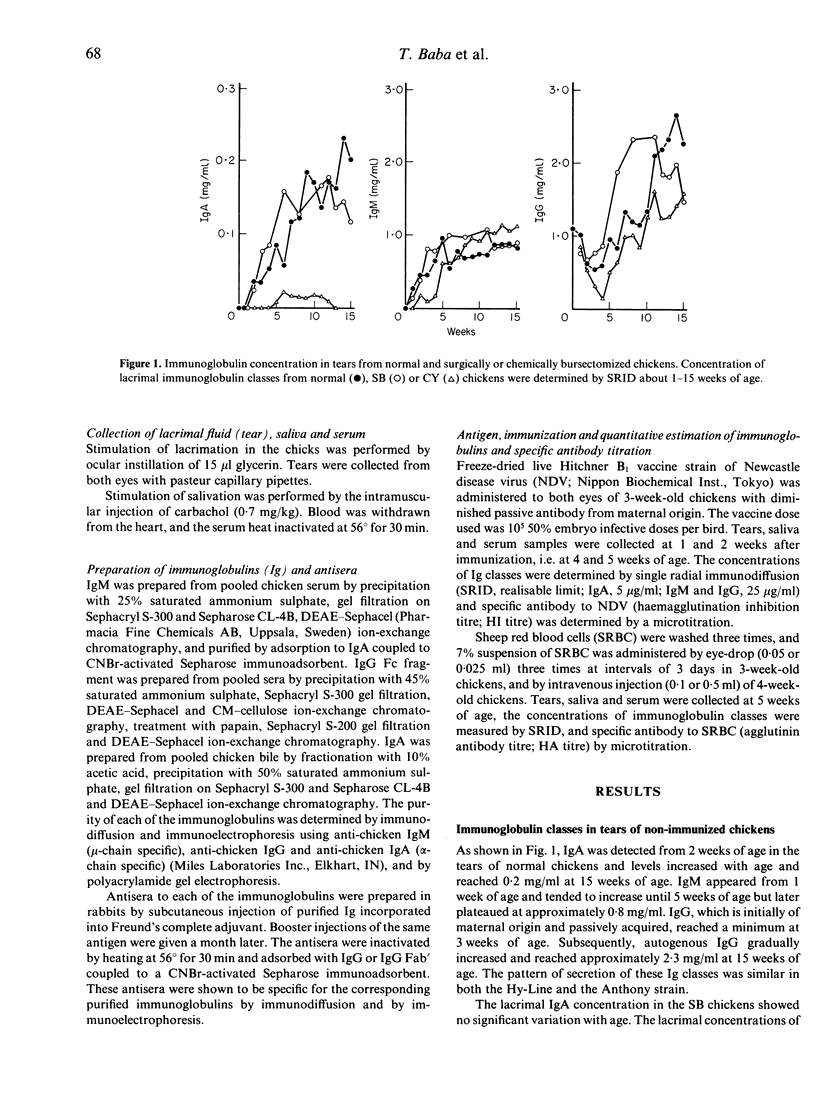
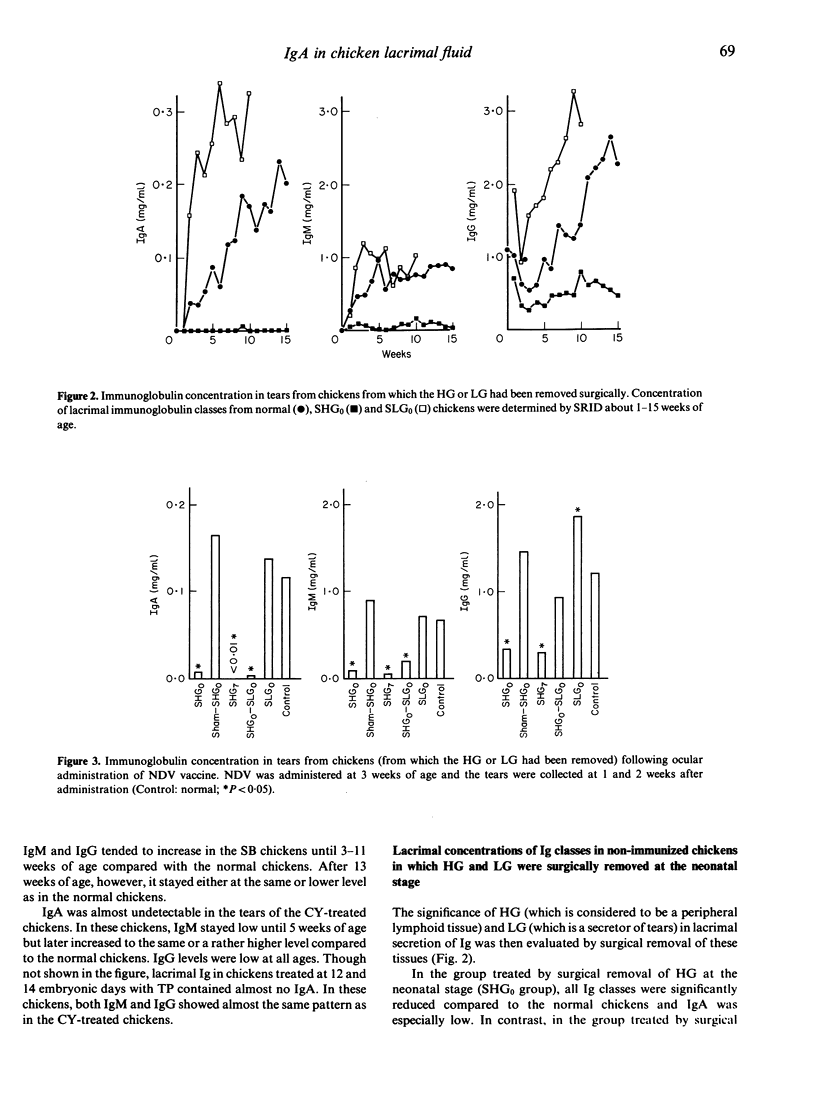
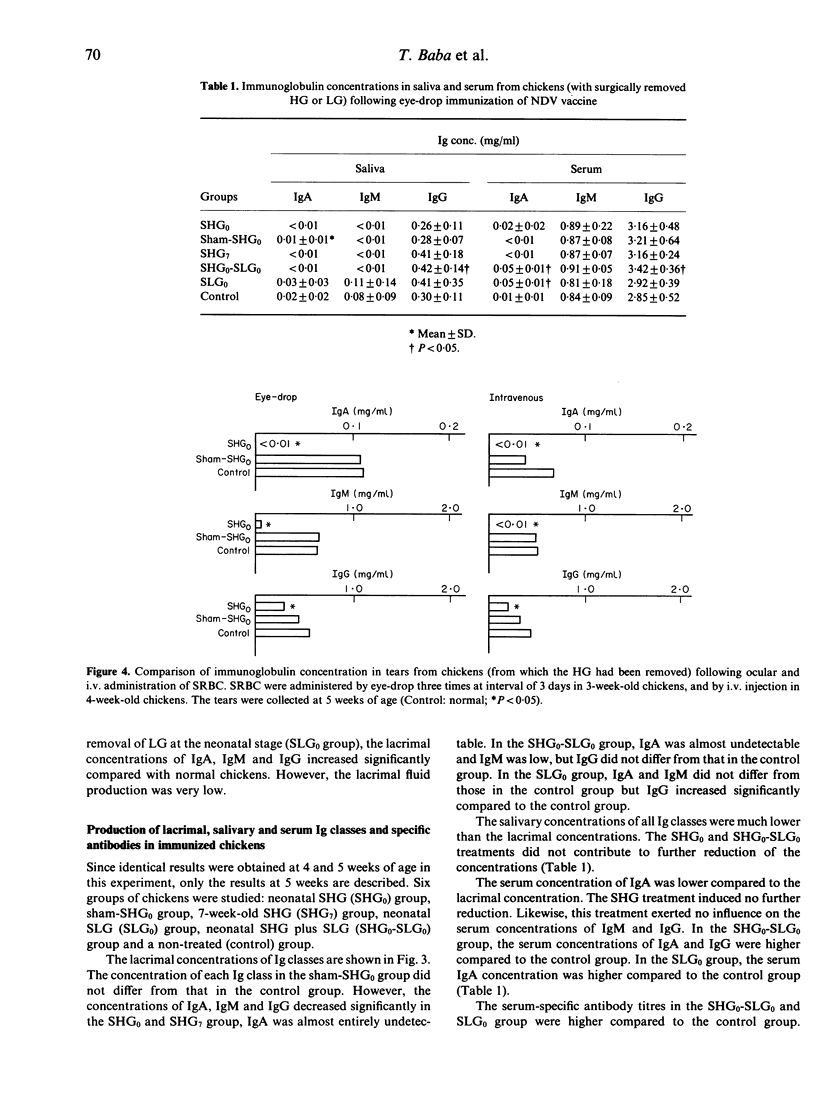
Involvement of the Harderian gland (HG) in the production of lacrimal immunoglobulin (especially IgA) was investigated. The lacrimal concentration of each immunoglobulin class was not affected by surgical bursectomy but was reduced by cyclophosphamide (CY) and testosterone (TP) treatments. Surgical removal of the Harderian gland caused a remarkable reduction of both the lacrimal concentration of each immunoglobulin class and the specific antibody titre, and and IgA was almost undetectable. The lacrimal concentration of each immunoglobulin class, as well as the specific antibody titre, was not affected by surgical removal of the Lacrimal gland (LG). The route of antigen administration produced no difference in the class of lacrimal immunoglobulin produced. The results indicate that the production of immunoglobulin in chicken tears may be dependent on the HG and that lacrimal immunoglobulin may be synthesized and secreted locally in the HG. Lymphocytes of the HG are of bursa of Fabricius origin and are seeded into the HG prior to hatching and its lymphocytes do not appear to be involved in systemic immunity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini B., Wick G. Delineation of B and T lymphoid cells in the chicken. J Immunol. 1974 Feb;112(2):444–450. [PubMed] [Google Scholar]
- Albini B., Wick G. Immunoglobulin determinants on the surface of chicken lymphoid cells. Int Arch Allergy Appl Immunol. 1973;44(6):804–822. doi: 10.1159/000230984. [DOI] [PubMed] [Google Scholar]
- Albini B., wick G. Ontogeny of lymphoid cell surface determinants in the chicken. Int Arch Allergy Appl Immunol. 1975;48(4):513–529. doi: 10.1159/000231339. [DOI] [PubMed] [Google Scholar]
- Bang B. G., Bang F. B. Localized lymphoid tissues and plasma cells in paraocular and paranasal organ systems in chickens. Am J Pathol. 1968 Nov;53(5):735–751. [PMC free article] [PubMed] [Google Scholar]
- Glick B., Rao D. S., Stinson R., McDuffie F. C. Immunoglobulin-positive cells from the gland of harder and bone marrow of the chicken. Cell Immunol. 1977 Jun 1;31(1):177–181. doi: 10.1016/0008-8749(77)90017-x. [DOI] [PubMed] [Google Scholar]
- Lebacq A. M., Ritter M. A. B-cell precursors in early chicken embryos. Immunology. 1979 May;37(1):123–134. [PMC free article] [PubMed] [Google Scholar]
- Lerner K. G., Glick B., McDuffie F. C. Role of the bursa of Fabricius in IgG and IgM production in the chicken: evidence for the role of a non-bursal site in the development of humoral immunity. J Immunol. 1971 Aug;107(2):493–503. [PubMed] [Google Scholar]
- Mueller A. P., Sato K., Glick B. The chicken lacrimal gland, gland of Harder, caecal tonsil, and accessory spleens as sources of antibody-producing cells. Cell Immunol. 1971 Apr;2(2):140–152. doi: 10.1016/0008-8749(71)90033-5. [DOI] [PubMed] [Google Scholar]
- Neumann U. Die chirurgische Entfernung der Harderschen Drüse des Huhnes. Zentralbl Veterinarmed A. 1976 May;23(04):323–330. [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. Source of IgA in tears of rats. Immunology. 1984 Dec;53(4):791–799. [PMC free article] [PubMed] [Google Scholar]


