Abstract
This study investigated the production of, and response to, the lymphokine interleukin-2 (IL-2) by functional subsets of human CD4+ T lymphocytes. Fresh peripheral blood mononuclear cells (PBMC) were sorted into CD4+2H4+/4B4- suppressor-inducer cells, and CD4+2H4-/4B4+ helper cells. The suppressor-inducer subset proliferated well in response to the T-cell mitogens concanavalin A (Con) A and phytohaemagglutinin (PHA), and produced IL-2. The helper cells produced no detectable IL-2 and proliferated poorly. However, the latter population were induced to express functional IL-2 receptors by Con A or purified protein derivative (PPD), and proliferated well if supplied with exogenous rIL-2. These findings suggest that the two functional CD4 subsets are not independent, or counteracting, but rather that the generation of T-cell help is likely to involve cooperative interactions between the two subsets.
Full text
PDF
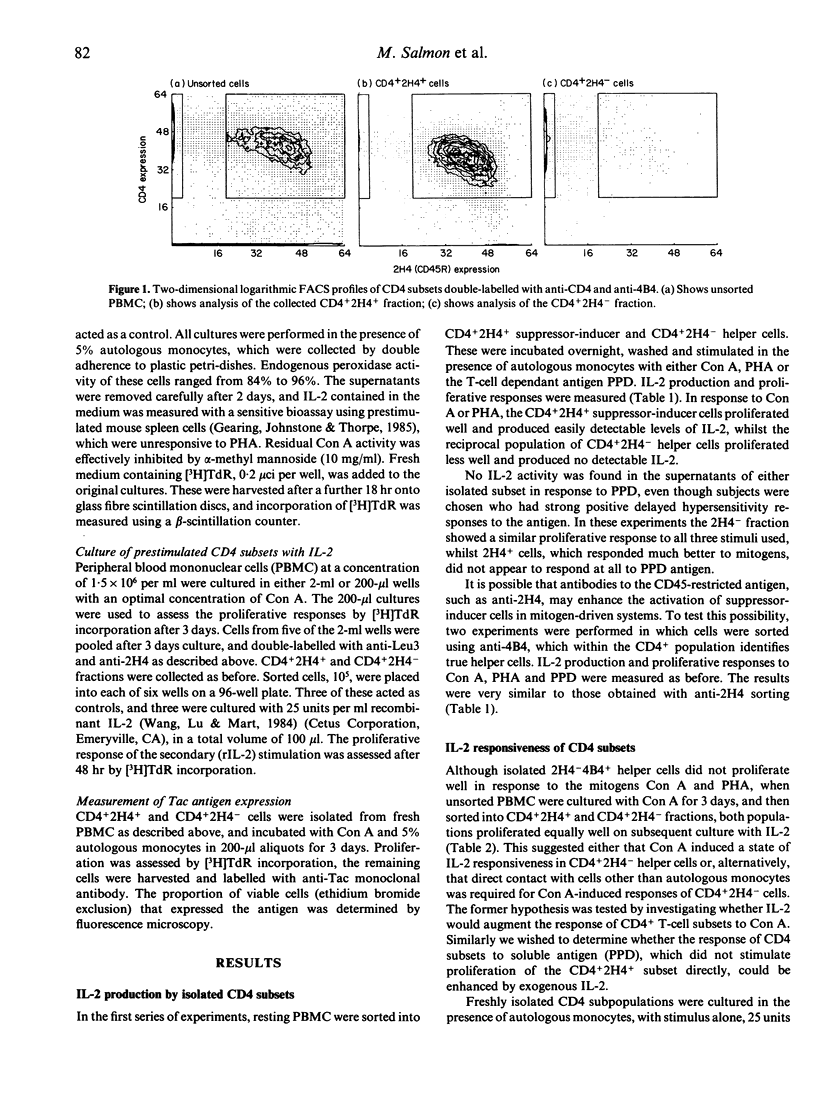
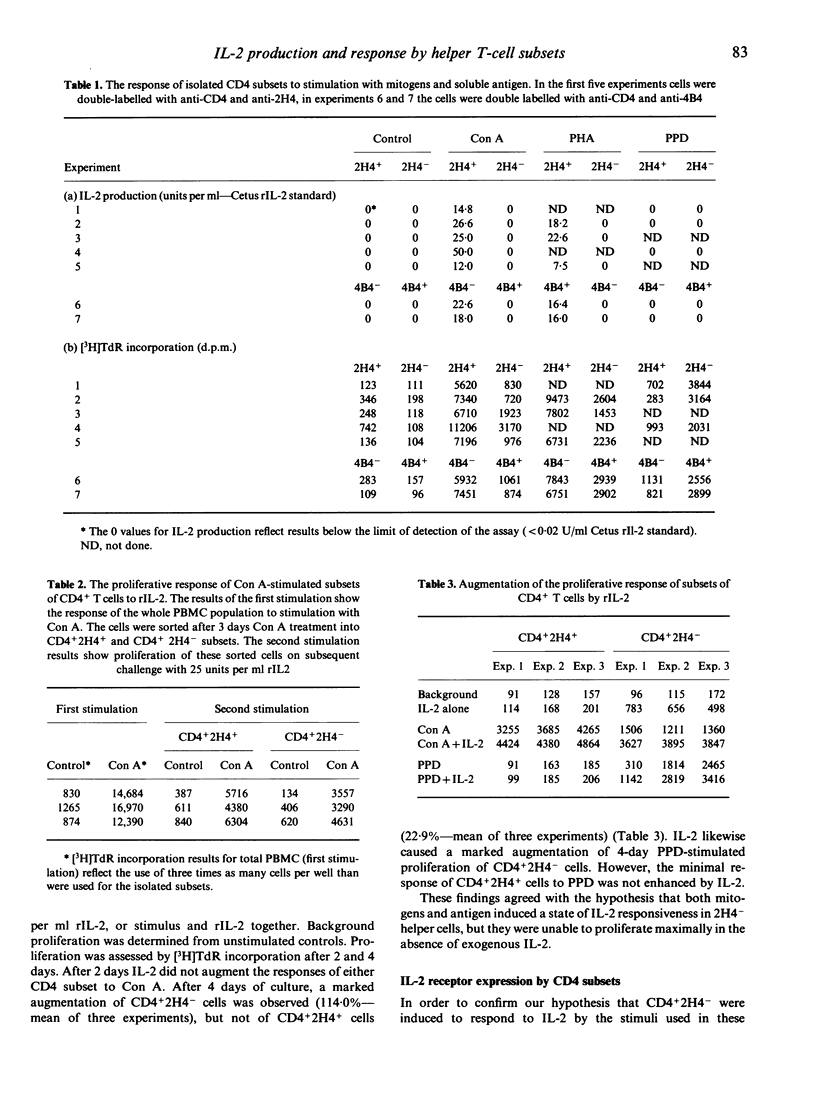
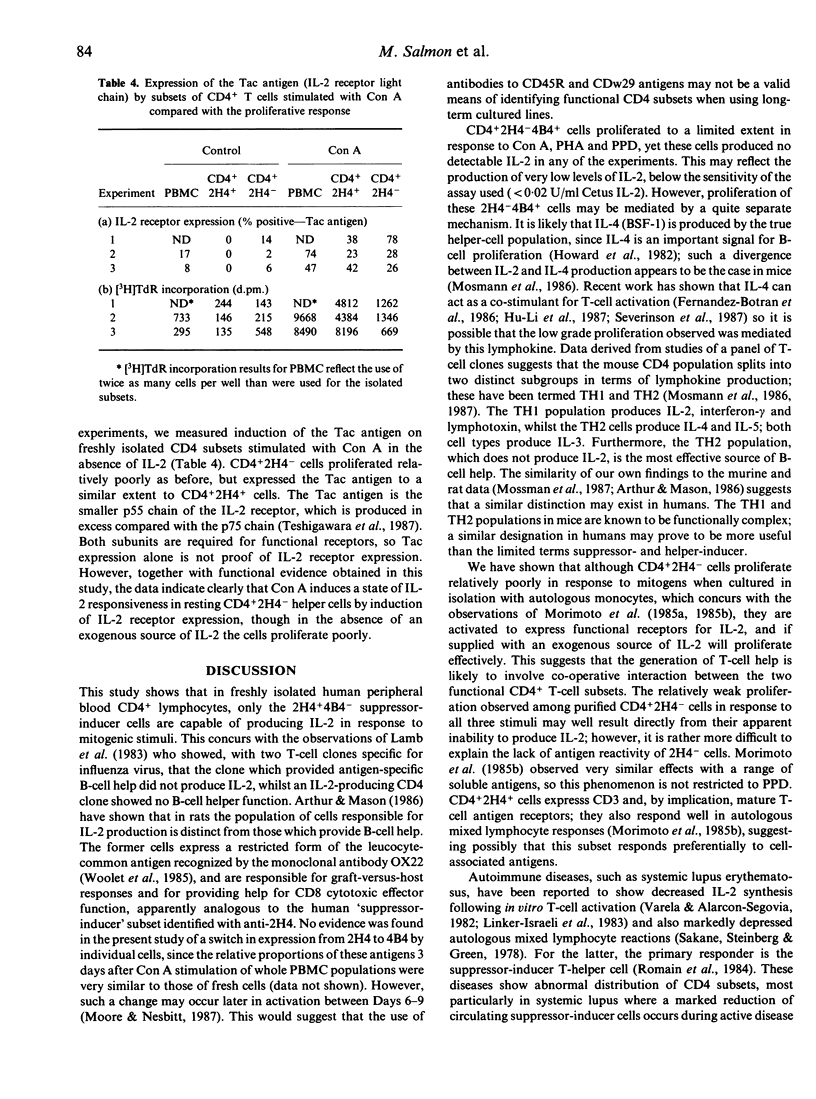

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Alcocer-Varela J., Alarcón-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982 Jun;69(6):1388–1392. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur R. P., Mason D. T cells that help B cell responses to soluble antigen are distinguishable from those producing interleukin 2 on mitogenic or allogeneic stimulation. J Exp Med. 1986 Apr 1;163(4):774–786. doi: 10.1084/jem.163.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing A. J., Johnstone A. P., Thorpe R. Production and assay of the interleukins. J Immunol Methods. 1985 Oct 24;83(1):1–27. doi: 10.1016/0022-1759(85)90053-5. [DOI] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Li J., Shevach E. M., Mizuguchi J., Ohara J., Mosmann T., Paul W. E. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J Exp Med. 1987 Jan 1;165(1):157–172. doi: 10.1084/jem.165.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Zanders E. D., Feldmann M., Lake P., Eckels D. D., Woody J. N., Beverley P. C. The dissociation of interleukin-2 production and antigen-specific helper activity by clonal analysis. Immunology. 1983 Nov;50(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- Luger T. A., Smolen J. S., Chused T. M., Steinberg A. D., Oppenheim J. J. Human lymphocytes with either the OKT4 or OKT8 phenotype produce interleukin 2 in culture. J Clin Invest. 1982 Aug;70(2):470–473. doi: 10.1172/JCI110637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K., Nesbitt A. M. Functional heterogeneity of CD4+ T lymphocytes: two subpopulations with counteracting immunoregulatory functions identified with the monoclonal antibodies WR16 and WR19. Immunology. 1987 Jun;61(2):159–165. [PMC free article] [PubMed] [Google Scholar]
- Moretta A. Frequency and surface phenotype of human T lymphocytes producing interleukin 2. Analysis by limiting dilution and cell cloning. Eur J Immunol. 1985 Feb;15(2):148–155. doi: 10.1002/eji.1830150208. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Morimoto C., Steinberg A. D., Letvin N. L., Hagan M., Takeuchi T., Daley J., Levine H., Schlossman S. F. A defect of immunoregulatory T cell subsets in systemic lupus erythematosus patients demonstrated with anti-2H4 antibody. J Clin Invest. 1987 Mar;79(3):762–768. doi: 10.1172/JCI112882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Romain P. L., Morimoto C., Daley J. F., Palley L. S., Reinherz E. L., Schlossman S. F. Reactivity of inducer cell subsets and T8-cell activation during the human autologous mixed lymphocyte reaction. Clin Immunol Immunopathol. 1984 Jan;30(1):117–128. doi: 10.1016/0090-1229(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Severinson E., Naito T., Tokumoto H., Fukushima D., Hirano A., Hama K., Honjo T. Interleukin 4 (IgG1 induction factor): a multifunctional lymphokine acting also on T cells. Eur J Immunol. 1987 Jan;17(1):67–72. doi: 10.1002/eji.1830170112. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Rudd C. E., Schlossman S. F., Morimoto C. Induction of suppression following autologous mixed lymphocyte reaction; role of a novel 2H4 antigen. Eur J Immunol. 1987 Jan;17(1):97–103. doi: 10.1002/eji.1830170117. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]


