Abstract
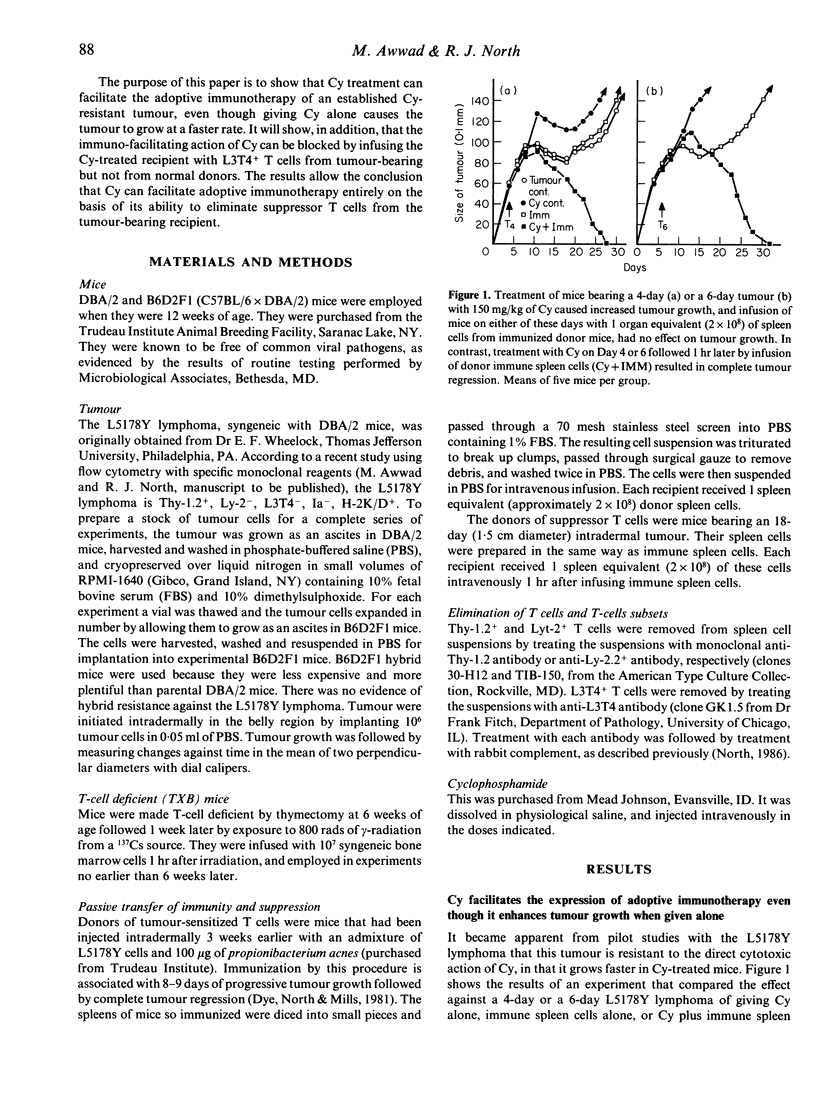
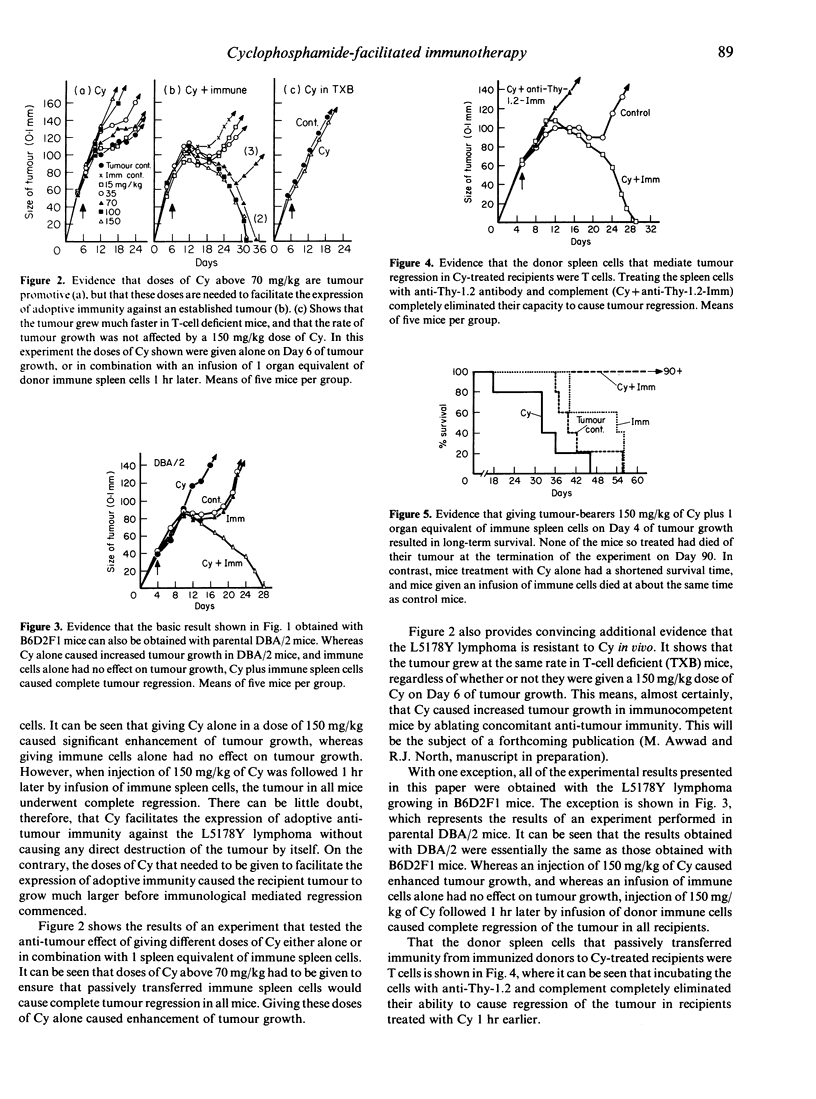
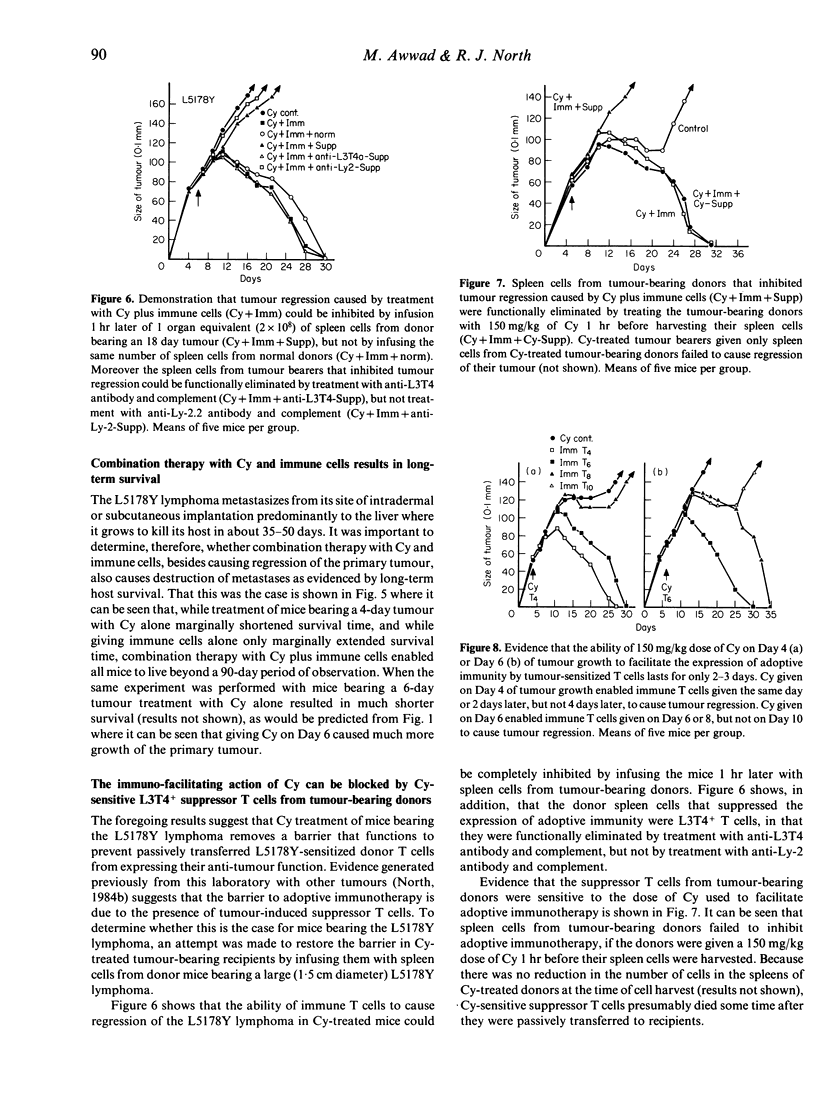
A cyclophosphamide (Cy)-resistant immunogenic tumour, the L5178Y lymphoma, was used to demonstrate that Cy-treatment of a host bearing this tumour enables passively transferred tumour-sensitized T cells to cause complete tumour regression without any need for Cy to cause a reduction in tumour burden. It was shown that whereas infusion of tumour-sensitized T cells from immune donors had very little effect on growth of the tumour, and whereas treatment with 150 mg/kg of Cy caused appreciable enhancement of tumour growth, combination therapy with Cy plus immune T cells caused complete tumour regression and resulted in long-term survival. Evidence that Cy treatment facilitated the expression of adoptive immunity against the L5178Y lymphoma by eliminating tumour-induced suppressor T cells consisted of the demonstration that tumour regression caused by combination treatment with Cy and immune T cells could be inhibited by infusing the recipient with Cy-sensitive, L3T4+ T cells from tumour-bearing but not from normal donors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt M. J., North R. J. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980 Jan 1;151(1):69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Nickol A. D., Ball E. J., Michael J. G., Bubel H. C. Development of protective immunity against bacterial and viral infections in tumor-bearing mice coincident with suppression of tumor immunity. J Reticuloendothel Soc. 1982 Jul;32(1):25–35. [PubMed] [Google Scholar]
- Bookman M. A., Swerdlow R., Matis L. A. Adoptive chemoimmunotherapy of murine leukemia with helper T lymphocyte clones. J Immunol. 1987 Nov 1;139(9):3166–3170. [PubMed] [Google Scholar]
- Claman H. N., Miller S. D., Conlon P. J., Moorhead J. W. Control of experimental contact sensitivity. Adv Immunol. 1980;30:121–157. doi: 10.1016/s0065-2776(08)60195-9. [DOI] [PubMed] [Google Scholar]
- Dye E. S., North R. J. Adoptive immunization against an established tumor with cytolytic versus memory T cells. Immediate versus delayed onset of regression. Transplantation. 1984 Jun;37(6):600–605. doi: 10.1097/00007890-198406000-00015. [DOI] [PubMed] [Google Scholar]
- Dye E. S., North R. J., Mills C. D. Mechanisms of anti-tumor action of Corynebacterium parvum. I. Potentiated tumor-specific immunity and its therapeutic limitations. J Exp Med. 1981 Sep 1;154(3):609–620. doi: 10.1084/jem.154.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. Combination therapy by using cyclophosphamide and tumor-sensitized lymphocytes: a possible mechanism of action. J Immunol. 1983 Jun;130(6):2511–2513. [PubMed] [Google Scholar]
- Goto M., Mitsuoka A., Sugiyama M., Kitano M. Enhancement of delayed hypersensitivity reaction with varieties of anti-cancer drugs. A common biological phenomenon. J Exp Med. 1981 Jul 1;154(1):204–209. doi: 10.1084/jem.154.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P. D., Cheever M. A. Treatment of disseminated leukemia with cyclophosphamide and immune cells: tumor immunity reflects long-term persistence of tumor-specific donor T cells. J Immunol. 1984 Dec;133(6):3401–3407. [PubMed] [Google Scholar]
- Greene M. I. The genetic and cellular basis of regulation of the immune response to tumor antigens. Contemp Top Immunobiol. 1980;11:81–116. doi: 10.1007/978-1-4684-3701-0_2. [DOI] [PubMed] [Google Scholar]
- Hall B. M., Jelbart M. E., Gurley K. E., Dorsch S. E. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. Mediation of specific suppression by T helper/inducer cells. J Exp Med. 1985 Nov 1;162(5):1683–1694. doi: 10.1084/jem.162.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Holán V., Mitchison N. A. Allospecific suppression without requirement for participation of Ly 2+ cells. Immunology. 1984 Mar;51(3):469–475. [PMC free article] [PubMed] [Google Scholar]
- Macphail S., Stutman O. Suppressor T cells activated in a primary in vitro response to non-major histocompatibility alloantigens. J Exp Med. 1982 Nov 1;156(5):1398–1414. doi: 10.1084/jem.156.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C. D., North R. J. Ly-1+2- suppressor T cells inhibit the expression of passively transferred antitumor immunity by suppressing the generation of cytolytic T cells. Transplantation. 1985 Feb;39(2):202–208. doi: 10.1097/00007890-198502000-00018. [DOI] [PubMed] [Google Scholar]
- North R. J., Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med. 1984 May 1;159(5):1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982 Apr 1;155(4):1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Down-regulation of the antitumor immune response. Adv Cancer Res. 1985;45:1–43. doi: 10.1016/s0065-230x(08)60265-1. [DOI] [PubMed] [Google Scholar]
- North R. J. Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother. 1984;16(3):175–181. doi: 10.1007/BF00205425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Models of adoptive T-cell-mediated regression of established tumors. Contemp Top Immunobiol. 1984;13:243–257. doi: 10.1007/978-1-4757-1445-6_12. [DOI] [PubMed] [Google Scholar]
- North R. J. Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation. Preferential elimination of suppressor T cells allows sustained production of effector T cells. J Exp Med. 1986 Nov 1;164(5):1652–1666. doi: 10.1084/jem.164.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Terry W. D. Passive immunotherapy of cancer in animals and man. Adv Cancer Res. 1977;25:323–388. doi: 10.1016/s0065-230x(08)60637-5. [DOI] [PubMed] [Google Scholar]
- Röllinghoff M., Starzinski-Powitz A., Pfizenmaier K., Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977 Feb 1;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Parker D. Effect of cyclophosphamide on immunological control mechanisms. Immunol Rev. 1982;65:99–113. doi: 10.1111/j.1600-065x.1982.tb00429.x. [DOI] [PubMed] [Google Scholar]


