Abstract
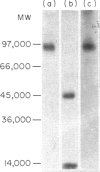
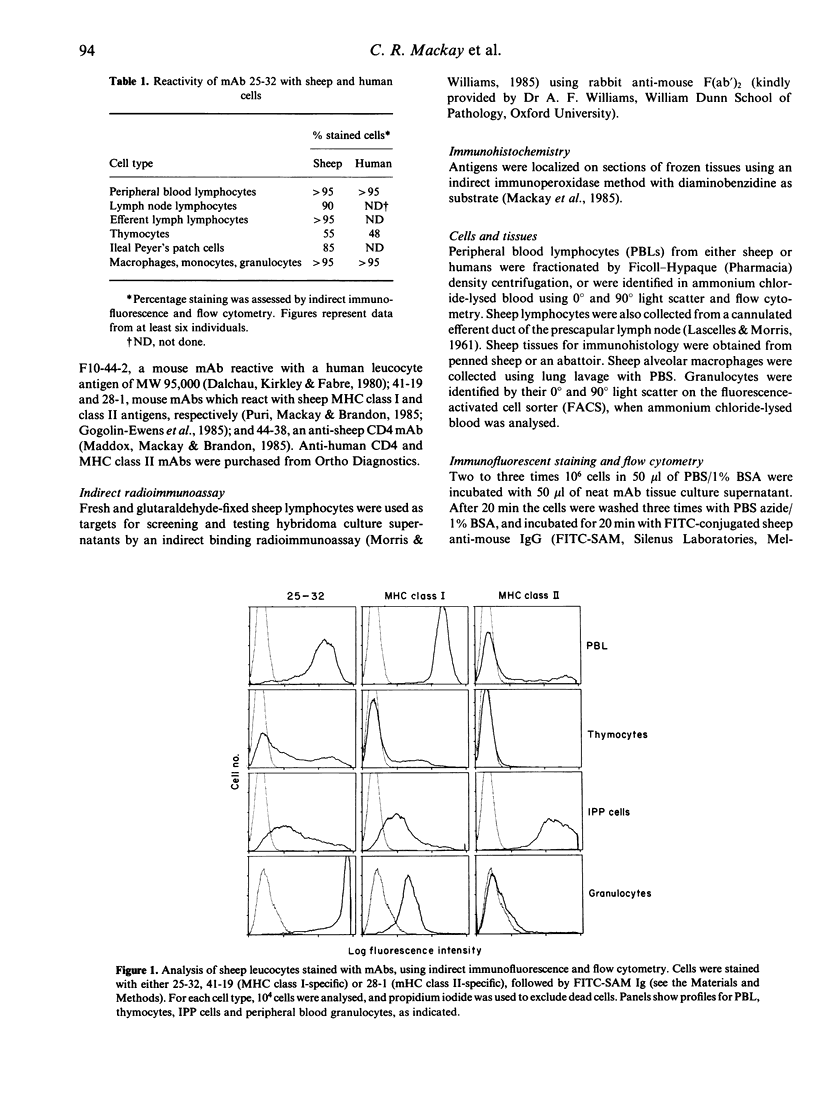
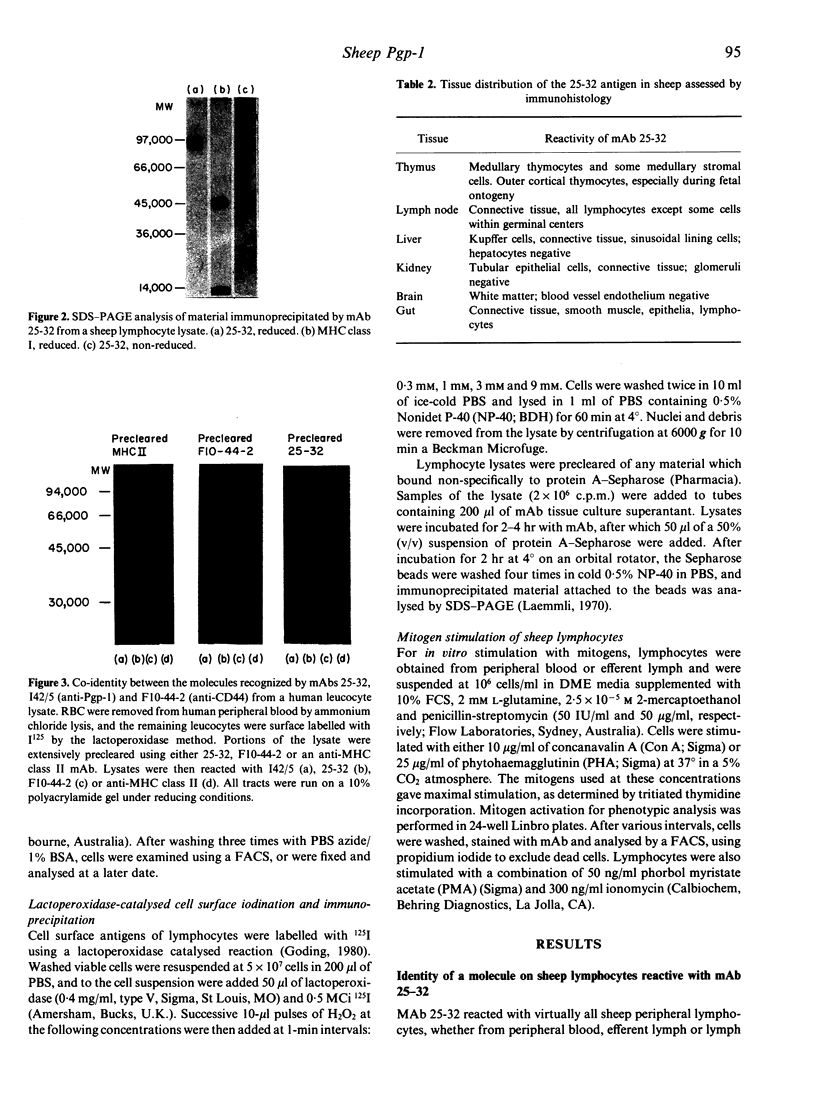
The phagocyte glycoprotein-1 (Pgp-1) antigen of mice is a 94,000 MW molecule with a wide tissue distribution, but no attributed function. We produced a monoclonal antibody (mAb) termed 25-32 which recognizes the Pgp-1 molecule of numerous mammalian species, including humans and sheep. Preclearing experiments with I42/5, a rat anti-mouse Pgp-1 mAb that cross-reacts with human Pgp-1, established the specificity of 25-32 for human and sheep Pgp-1. Moreover, an antibody recognizing human CD44, termed F10-44-2, also reacted with the same molecule as that recognized by 25-32 and I42/5, so establishing the co-identity of CD44 and Pgp-1. Within the sheep thymus, Pgp-1 was expressed most strongly by medullary thymocytes and stromal cells, and by small numbers of cells at the subcapsular cortex. Pgp-1 was expressed early in thymic ontogeny; all 35-40-day gestation fetal sheep thymocytes were intensely Pgp-1+, but by 80 days the number of reactive thymocytes had decreased to adult levels. The expression of Pgp-1 on lymphocytes was markedly increased after stimulation with mitogens, or with phorbol esters and ionomycin. The highly conserved nature of Pgp-1 through evolution, its expression on virtually all cell types within the body, and its increased expression on rapidly dividing cells indicate that this molecule mediates an important function, possibly serving as a hormone or metabolite receptor.
Full text
PDF
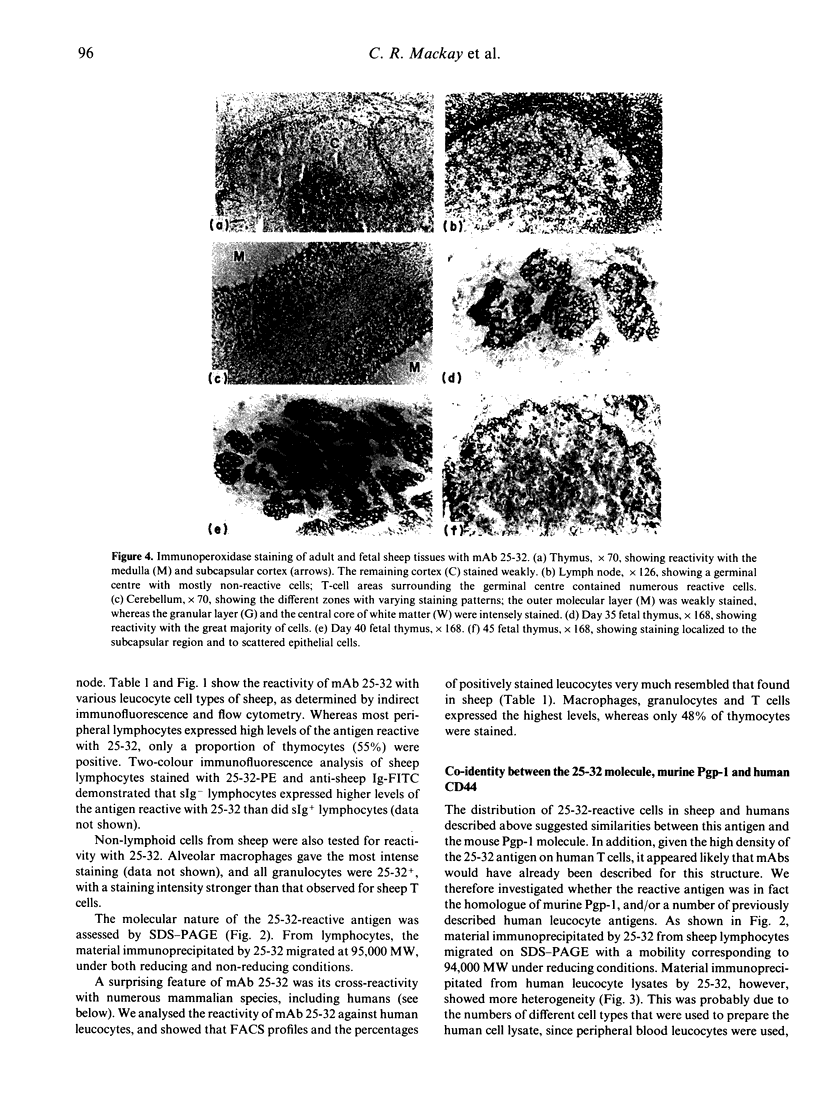
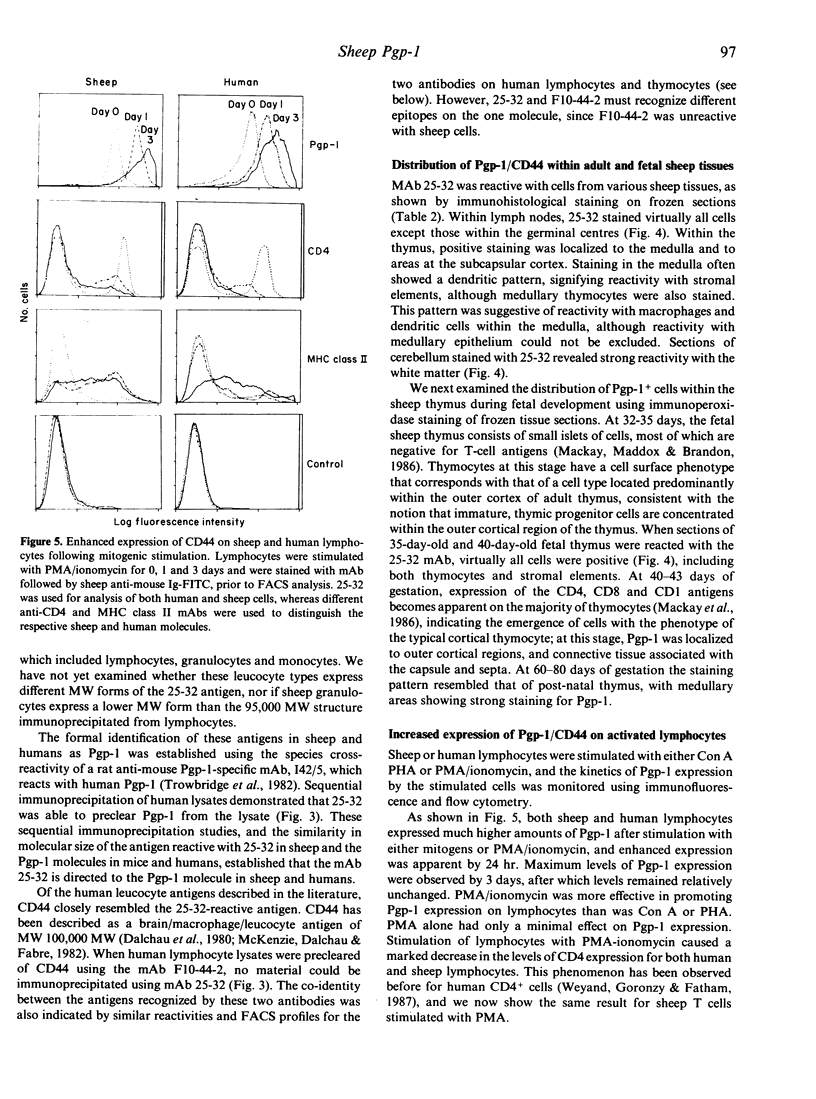


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budd R. C., Cerottini J. C., Horvath C., Bron C., Pedrazzini T., Howe R. C., MacDonald H. R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987 May 15;138(10):3120–3129. [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Phenotypic identification of memory cytolytic T lymphocytes in a subset of Lyt-2+ cells. J Immunol. 1987 Feb 15;138(4):1009–1013. [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Selectively increased production of interferon-gamma by subsets of Lyt-2+ and L3T4+ T cells identified by expression of Pgp-1. J Immunol. 1987 Jun 1;138(11):3583–3586. [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- Gogolin-Ewens K. J., Mackay C. R., Mercer W. R., Brandon M. R. Sheep lymphocyte antigens (OLA). I. Major histocompatibility complex class I molecules. Immunology. 1985 Dec;56(4):717–723. [PMC free article] [PubMed] [Google Scholar]
- Isacke C. M., Sauvage C. A., Hyman R., Lesley J., Schulte R., Trowbridge I. S. Identification and characterization of the human Pgp-1 glycoprotein. Immunogenetics. 1986;23(5):326–332. doi: 10.1007/BF00398797. [DOI] [PubMed] [Google Scholar]
- LASCELLES A. K., MORRIS B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Q J Exp Physiol Cogn Med Sci. 1961 Jul;46:199–205. doi: 10.1113/expphysiol.1961.sp001536. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Schulte R. Evidence that the Pgp-1 glycoprotein is expressed on thymus-homing progenitor cells of the thymus. Cell Immunol. 1985 Apr 1;91(2):397–403. doi: 10.1016/0008-8749(85)90237-0. [DOI] [PubMed] [Google Scholar]
- Lesley J., Trotter J., Hyman R. The Pgp-1 antigen is expressed on early fetal thymocytes. Immunogenetics. 1985;22(2):149–157. doi: 10.1007/BF00563512. [DOI] [PubMed] [Google Scholar]
- Lesley J., Trowbridge I. S. Genetic characterization of a polymorphic murine cell-surface glycoprotein. Immunogenetics. 1982 Mar;15(3):313–320. doi: 10.1007/BF00364339. [DOI] [PubMed] [Google Scholar]
- Lynch F., Chaudhri G., Allan J. E., Doherty P. C., Ceredig R. Expression of Pgp-1 (or Ly24) by subpopulations of mouse thymocytes and activated peripheral T lymphocytes. Eur J Immunol. 1987 Jan;17(1):137–140. doi: 10.1002/eji.1830170123. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Brandon M. R. Thymocyte subpopulations during early fetal development in sheep. J Immunol. 1986 Mar 1;136(5):1592–1599. [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Gogolin-Ewens K. J., Brandon M. R. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology. 1985 Aug;55(4):729–737. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. The sheep analogue of leucocyte common antigen (LCA). Immunology. 1985 Jun;55(2):347–353. [PMC free article] [PubMed] [Google Scholar]
- McKenzie J. L., Dalchau R., Fabre J. W. Biochemical characterisation and localization in brain of a human brain-leucocyte membrane glycoprotein recognised by a monoclonal antibody. J Neurochem. 1982 Nov;39(5):1461–1466. doi: 10.1111/j.1471-4159.1982.tb12592.x. [DOI] [PubMed] [Google Scholar]
- Morris R. J., Williams A. F. Antigens on mouse and rat lymphocytes recognized by rabbit antiserum against rat brain: the quantitative analysis of a xenogeneic antiserum. Eur J Immunol. 1975 Apr;5(4):274–281. doi: 10.1002/eji.1830050412. [DOI] [PubMed] [Google Scholar]
- Puri N. K., Mackay C. R., Brandon M. R. Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules. Immunology. 1985 Dec;56(4):725–733. [PMC free article] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton V. R., Wijffels G. L., Walker I. D., Hogarth P. M., McKenzie I. F. Genetic and biochemical characterization of antigens encoded by the Ly-24 (Pgp-1) locus. J Immunogenet. 1987 Feb;14(1):43–57. doi: 10.1111/j.1744-313x.1987.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Schulte R., Hyman R., Trotter J. Biochemical characterization and cellular distribution of a polymorphic, murine cell-surface glycoprotein expressed on lymphoid tissues. Immunogenetics. 1982 Mar;15(3):299–312. doi: 10.1007/BF00364338. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Trotter J., Hyman R. Thymocyte subpopulation enriched for progenitors with an unrearranged T-cell receptor beta-chain gene. Nature. 1985 Jun 20;315(6021):666–669. doi: 10.1038/315666a0. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J., Fathman C. G. Modulation of CD4 by antigenic activation. J Immunol. 1987 Mar 1;138(5):1351–1354. [PubMed] [Google Scholar]