Abstract
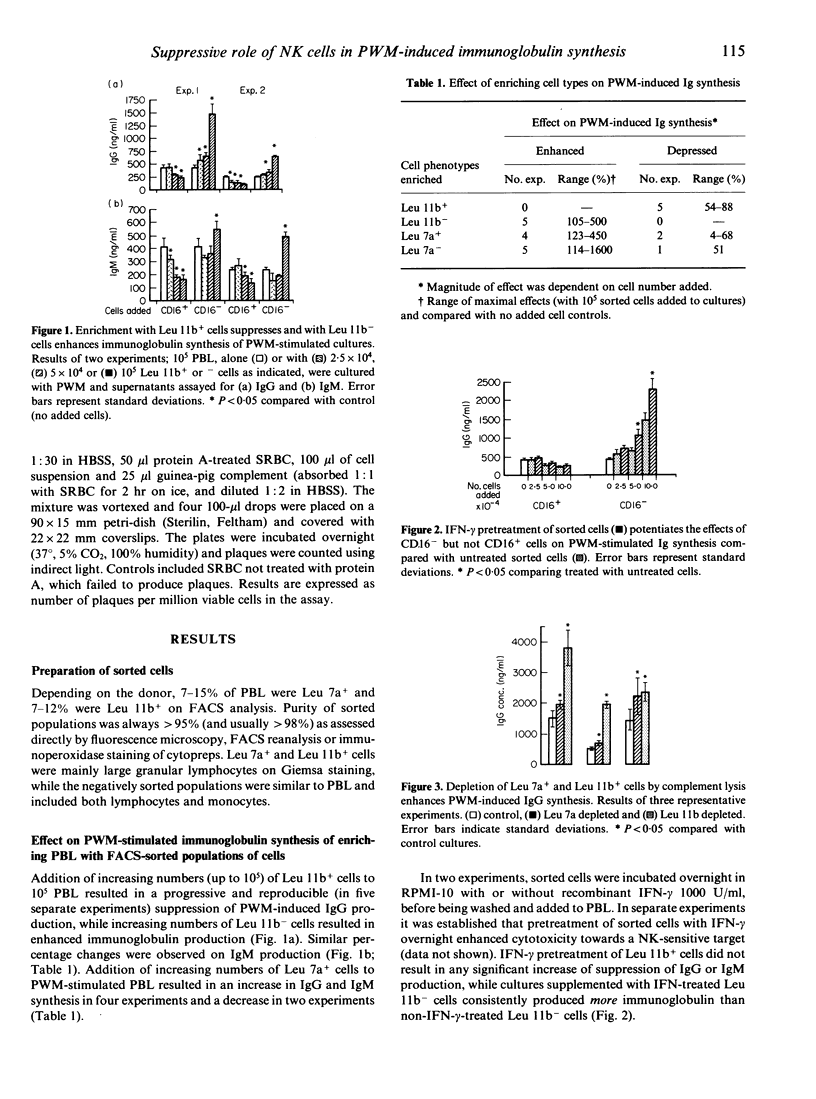
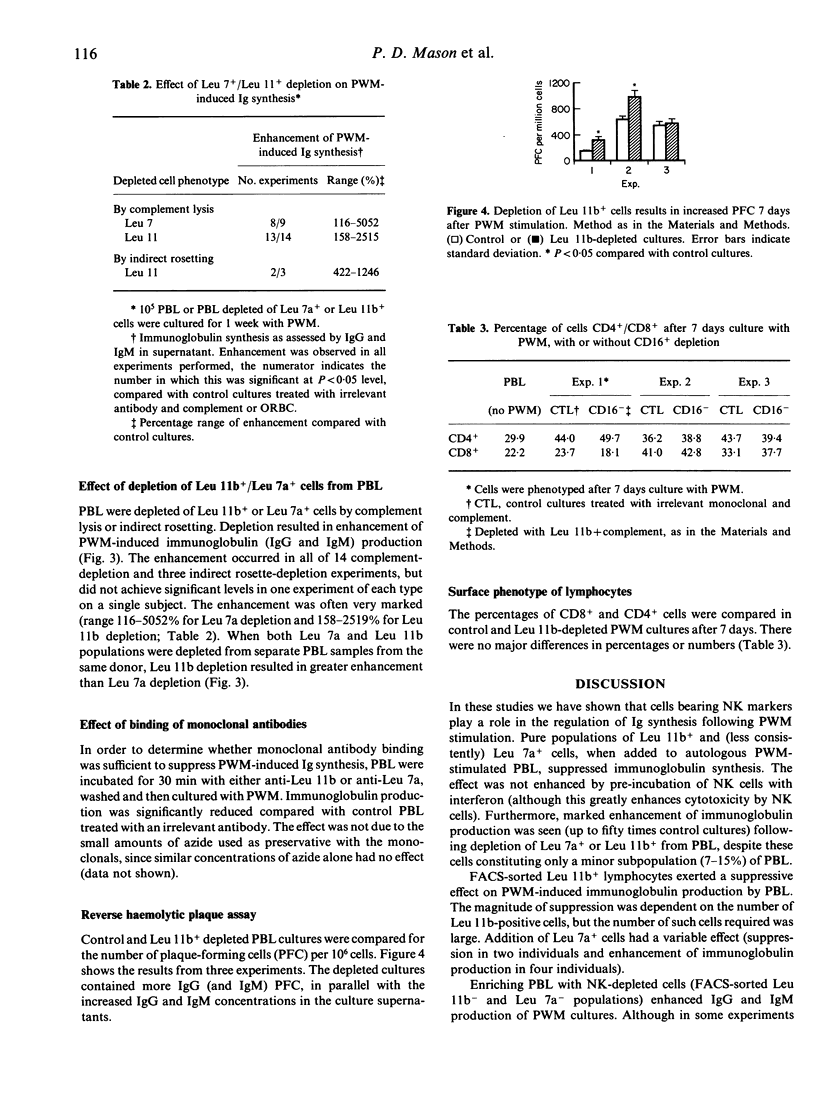
Natural killer (NK) cells probably have immunoregulatory effects. However, the evidence to date is mainly based on the suppressive effect of enrichment with relatively impure NK populations (large granular lymphocytes, LGL, Leu 7a+ cells). Here we report on the effect of enrichment and depletion of Leu 7a+ and Leu 11b+ cells (the latter containing virtually all NK activity in freshly prepared lymphocytes) on pokeweed mitogen (PWM)-induced immunoglobulin (Ig) synthesis. Enrichment suppressed Ig synthesis to a degree dependent on the number of cells added, and was not enhanced further by their pretreatment with interferon. Furthermore, depletion of Leu 11b+ cells from peripheral blood lymphocytes (PBL) led to marked enhancement (2-25-fold increase) of Ig synthesis, suggesting these cells may normally exert a suppressive effect. The possible underlying mechanisms were investigated further. Enhanced Ig synthesis by Leu 11b-depleted cultures was associated with an increased number of Ig-secreting cells by plaque assay, but with no change in numbers of CD4+ or CD8+ cells. Treatment of PBL with monoclonal antibodies (anti-Leu 7a/Leu 11b) alone suppressed PWM-induced immunoglobulin synthesis. We conclude that NK cells play a role in the regulation of Ig production, at least in part by an effect on activation/differentiation of B cells, but independent of altered T-cell subpopulations. The effect may be unrelated to their cytotoxic function (being unaffected by interferon, IFN), although the direct effects of anti-Leu 11b and Leu 7a in enhancing the suppressive effect suggest an alternative activation pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Arai S., Yamamoto H., Itoh K., Kumagai K. Suppressive effect of human natural killer cells on pokeweed mitogen-induced B cell differentiation. J Immunol. 1983 Aug;131(2):651–657. [PubMed] [Google Scholar]
- Brenner M. K., Reittie J. E., Grob J. P., Wimperis J. Z., Stephens S., Patterson J., Hoffbrand A. V., Prentice H. G. The contribution of large granular lymphocytes to B cell activation and differentiation after T-cell-depleted allogeneic bone marrow transplantation. Transplantation. 1986 Sep;42(3):257–261. doi: 10.1097/00007890-198609000-00006. [DOI] [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J Immunol. 1984 Feb;132(2):611–615. [PubMed] [Google Scholar]
- Degliantoni G., Perussia B., Mangoni L., Trinchieri G. Inhibition of bone marrow colony formation by human natural killer cells and by natural killer cell-derived colony-inhibiting activity. J Exp Med. 1985 May 1;161(5):1152–1168. doi: 10.1084/jem.161.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Beran M., Andersson B., Kiessling R. Inhibition of in vitro granulopoiesis by autologous allogeneic human NK cells. J Immunol. 1982 Jul;129(1):126–132. [PubMed] [Google Scholar]
- Hansson M., Kiessling R., Andersson B. Human fetal thymus and bone marrow contain target cells for natural killer cells. Eur J Immunol. 1981 Jan;11(1):8–12. doi: 10.1002/eji.1830110103. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Hochman P. S., Haller O., Shearer G. M., Wigzell H., Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977 Sep;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Krensky A. M., Lanier L. L., Engleman E. G. Lymphocyte subsets and surface molecules in man. Clin Immunol Rev. 1985;4(1):95–138. [PubMed] [Google Scholar]
- Kuwano K., Arai S., Munakata T., Tomita Y., Yoshitake Y., Kumagai K. Suppressive effect of human natural killer cells on Epstein-Barr virus-induced immunoglobulin synthesis. J Immunol. 1986 Sep 1;137(5):1462–1468. [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Leibson H. J., Gefter M., Zlotnik A., Marrack P., Kappler J. W. Role of gamma-interferon in antibody-producing responses. 1984 Jun 28-Jul 4Nature. 309(5971):799–801. doi: 10.1038/309799a0. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Pollack S. B. Prevention of rejection of allogeneic bone marrow transplants by NK 1.1 antiserum. Transplantation. 1983 May;35(5):490–494. doi: 10.1097/00007890-198305000-00019. [DOI] [PubMed] [Google Scholar]
- Masucci M. G., Bejarano M. T., Masucci G., Klein E. Large granular lymphocytes inhibit the in vitro growth of autologous Epstein-Barr virus-infected B cells. Cell Immunol. 1983 Mar;76(2):311–321. doi: 10.1016/0008-8749(83)90374-x. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Phillips J. H., Warner N. L., Lanier L. L. Correlation of biophysical properties and cell surface antigenic profile of Percoll gradient-separated human natural killer cells. Nat Immun Cell Growth Regul. 1983;3(2):73–86. [PubMed] [Google Scholar]
- Pistoia V., Cozzolino F., Torcia M., Castigli E., Ferrarini M. Production of B cell growth factor by a Leu-7+, OKM1+ non-T cell with the features of large granular lymphocytes (LGL). J Immunol. 1985 May;134(5):3179–3184. [PubMed] [Google Scholar]
- Puck J. M., Rich R. R. Regulatory interactions governing the proliferation of T cell subsets stimulated with pokeweed mitogen. J Immunol. 1984 Mar;132(3):1106–1112. [PubMed] [Google Scholar]
- Shope T. C., Kaplan J. Inhibition of the in vitro outgrowth of Epstein-Barr virus-infected lymphocytes by TG lymphocytes. J Immunol. 1979 Nov;123(5):2150–2155. [PubMed] [Google Scholar]
- Targan S., Brieva J., Newman W., Stevens R. Is the NK lytic process involved in the mechanism of NK suppression of antibody-producing cells? J Immunol. 1985 Feb;134(2):666–669. [PubMed] [Google Scholar]
- Thomas Y., Sosman J., Irigoyen O., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. I. Collaborative T-T interactions in the immunoregulation of B cell differentiation. J Immunol. 1980 Dec;125(6):2402–2408. [PubMed] [Google Scholar]
- Thomas Y., Sosman J., Rogozinski L., Irigoyen O., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. III. Regulation of helper factor production by T cell subsets. J Immunol. 1981 May;126(5):1948–1951. [PubMed] [Google Scholar]
- Tilden A. B., Abo T., Balch C. M. Suppressor cell function of human granular lymphocytes identified by the HNK-1 (Leu 7) monoclonal antibody. J Immunol. 1983 Mar;130(3):1171–1175. [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Vyakarnam A., Brenner M. K., Reittie J. E., Houlker C. H., Lachmann P. J. Human clones with natural killer function can activate B cells and secrete B cell differentiation factors. Eur J Immunol. 1985 Jun;15(6):606–610. doi: 10.1002/eji.1830150614. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Volkman D. J., Burman K. D., Fauci A. S. Activation, proliferation, and differentiation of circulating B cells in autoimmune thyroid disease. J Immunol. 1985 Nov;135(5):3138–3143. [PubMed] [Google Scholar]


