Abstract
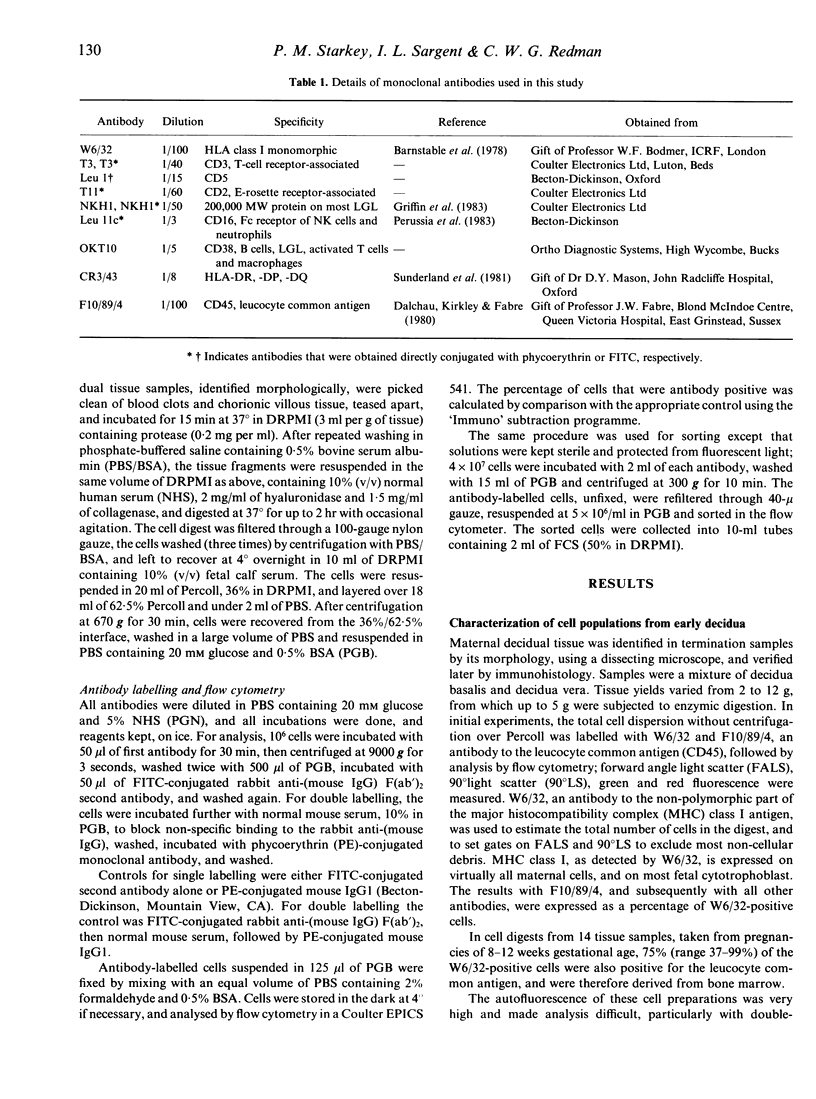
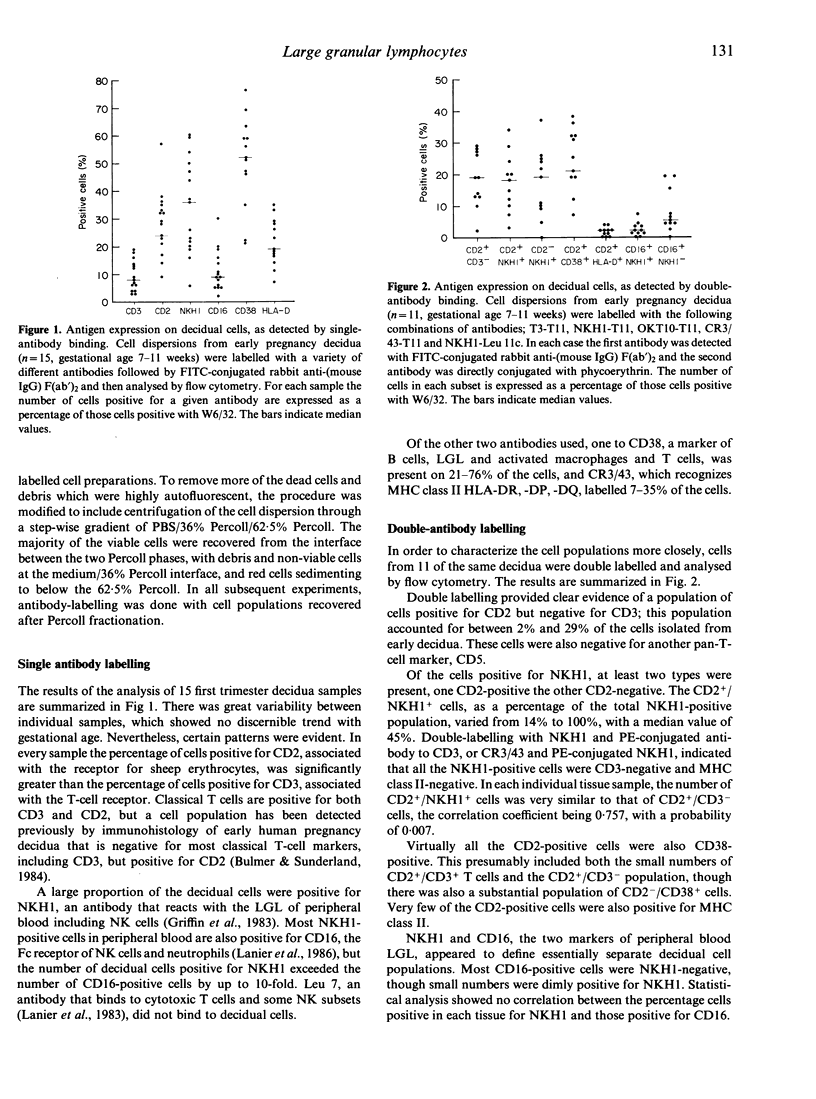
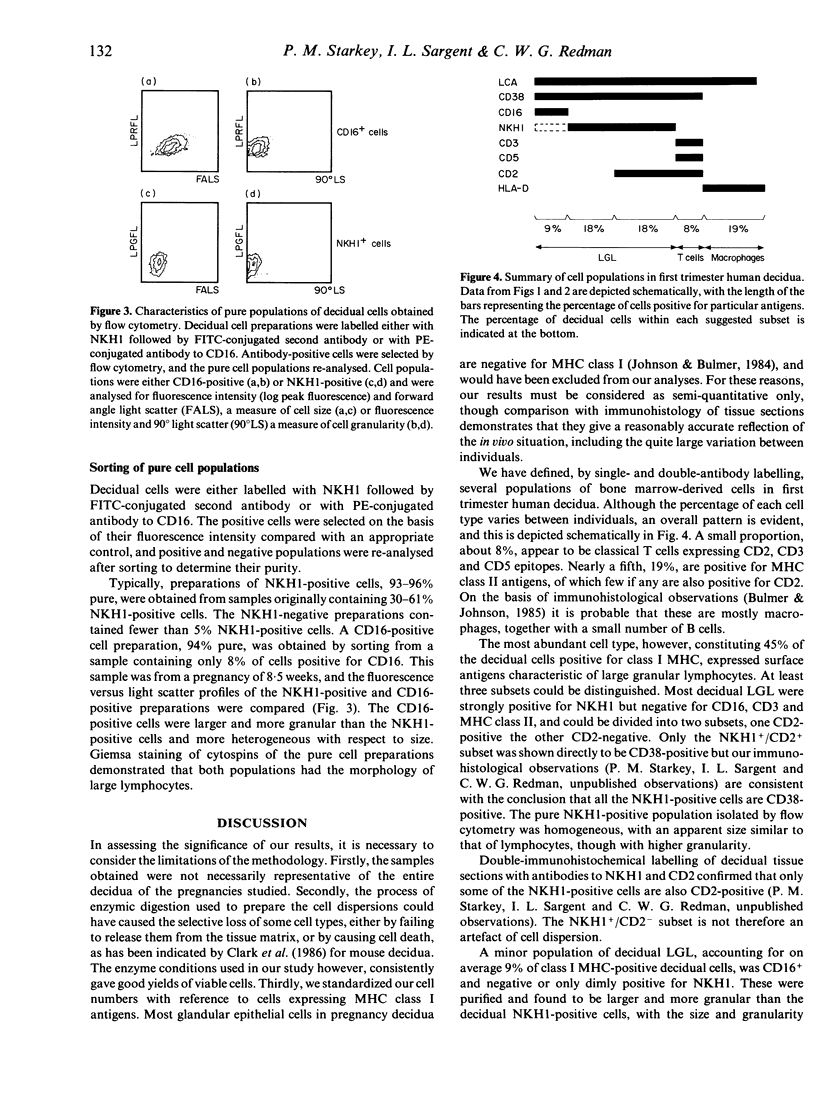
Cell populations of human pregnancy decidua, obtained by enzymic digestion from first trimester samples, were analysed by flow cytometry after labelling with monoclonal antibodies. The majority of these decidual cells (75%) were of bone marrow origin. The most abundant cell type expressed antigens characteristic of large granular lymphocytes (LGL), although macrophages and small numbers of classical T cells were also present. Three subsets of decidual LGL can be defined by single-and double-antibody labelling. Most decidual LGL are positive for NKH1, a marker of peripheral blood LGL, but negative for CD16, the Fc receptor of NK cells, and for the T-cell markers CD3 and CD5. About half the NKH1-positive cells also express CD2, associated with the E-rosette receptor, and are identical to the CD3-negative/CD2-positive cells reported previously in early pregnancy decidua. The NKH1-positive cells apparently correspond to a minor subset of peripheral blood LGL. The remaining decidual LGL are positive for CD16 and negative or only dimly positive for NKH1, and are similar to the major type of peripheral blood LGL. After purification by flow cytometry, the NKH1-positive cells were demonstrated to be of similar size to, but slightly higher granularity than, lymphocytes, whereas the CD16-positive cells were larger and more granular. The possible role of decidual LGL in modulating placental development is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Arai S., Yamamoto H., Itoh K., Kumagai K. Suppressive effect of human natural killer cells on pokeweed mitogen-induced B cell differentiation. J Immunol. 1983 Aug;131(2):651–657. [PubMed] [Google Scholar]
- Athanassakis I., Bleackley R. C., Paetkau V., Guilbert L., Barr P. J., Wegmann T. G. The immunostimulatory effect of T cells and T cell lymphokines on murine fetally derived placental cells. J Immunol. 1987 Jan 1;138(1):37–44. [PubMed] [Google Scholar]
- Bulmer J. N., Johnson P. M. Macrophage populations in the human placenta and amniochorion. Clin Exp Immunol. 1984 Aug;57(2):393–403. [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Sunderland C. A. Immunohistological characterization of lymphoid cell populations in the early human placental bed. Immunology. 1984 Jun;52(2):349–357. [PMC free article] [PubMed] [Google Scholar]
- Chatterjee-Hasrouni S., Santer V., Lala P. K. Characterization of maternal small lymphocyte subsets during allogeneic pregnancy in the mouse. Cell Immunol. 1980 Mar 15;50(2):290–304. doi: 10.1016/0008-8749(80)90284-1. [DOI] [PubMed] [Google Scholar]
- Clark D. A., Slapsys R. M., Croy B. A., Rossant J. Suppressor cell activity in uterine decidua correlates with success or failure of murine pregnancies. J Immunol. 1983 Aug;131(2):540–542. [PubMed] [Google Scholar]
- Clark D. A., Slapsys R., Croy B. A., Krcek J., Rossant J. Local active suppression by suppressor cells in the decidua: a review. Am J Reprod Immunol. 1984 Mar;5(2):78–83. doi: 10.1111/j.1600-0897.1984.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Croy B. A., Gambel P., Rossant J., Wegmann T. G. Characterization of murine decidual natural killer (NK) cells and their relevance to the success of pregnancy. Cell Immunol. 1985 Jul;93(2):315–326. doi: 10.1016/0008-8749(85)90137-6. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Daya S., Clark D. A., Devlin C., Jarrell J., Chaput A. Suppressor cells in human decidua. Am J Obstet Gynecol. 1985 Jan 15;151(2):267–270. doi: 10.1016/0002-9378(85)90024-9. [DOI] [PubMed] [Google Scholar]
- Daya S., Clark D. A., Devlin C., Jarrell J. Preliminary characterization of two types of suppressor cells in the human uterus. Fertil Steril. 1985 Dec;44(6):778–785. doi: 10.1016/s0015-0282(16)49037-0. [DOI] [PubMed] [Google Scholar]
- Gambel P., Croy B. A., Moore W. D., Hunziker R. D., Wegmann T. G., Rossant J. Characterization of immune effector cells present in early murine decidua. Cell Immunol. 1985 Jul;93(2):303–314. doi: 10.1016/0008-8749(85)90136-4. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Atkinson M. E. Large agranular lymphocytes: early non-specific effector cells in allograft rejection in the mouse. Immunology. 1984 Oct;53(2):257–265. [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Hercend T., Beveridge R., Schlossman S. F. Characterization of an antigen expressed by human natural killer cells. J Immunol. 1983 Jun;130(6):2947–2951. [PubMed] [Google Scholar]
- Johnson P. M., Bulmer J. N. Uterine gland epithelium in human pregnancy often lacks detectable maternal MHC antigens but does express fetal trophoblast antigens. J Immunol. 1984 Apr;132(4):1608–1610. [PubMed] [Google Scholar]
- Kearns M., Lala P. K. Characterization of hematogenous cellular constituents of the murine decidua: a surface marker study. J Reprod Immunol. 1985 Nov;8(2-3):213–234. doi: 10.1016/0165-0378(85)90042-7. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Nakayama E., Asano S., Kodo H., Miwa S. Suppression of mixed lymphocyte reaction by cells of human first trimester pregnancy endometrium. J Reprod Immunol. 1985 Aug;8(1):25–31. doi: 10.1016/0165-0378(85)90075-0. [DOI] [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Ritson A., Bulmer J. N. Endometrial granulocytes in human decidua react with a natural-killer (NK) cell marker, NKH1. Immunology. 1987 Oct;62(2):329–331. [PMC free article] [PubMed] [Google Scholar]
- Slapsys R. M., Richards C. D., Clark D. A. Active suppression of host-versus-graft reaction in pregnant mice. VIII. The uterine decidua-associated suppressor cell is distinct from decidual NK cells. Cell Immunol. 1986 Apr 15;99(1):140–149. doi: 10.1016/0008-8749(86)90223-6. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., Naiem M., Mason D. Y., Redman C. W., Stirrat G. M. The expression of major histocompatibility antigens by human chorionic villi. J Reprod Immunol. 1981 Dec;3(6):323–331. doi: 10.1016/0165-0378(81)90048-6. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Baines M. G. Modulation of the natural killer cell activity in pregnant mice alters the spontaneous abortion rate. J Reprod Immunol. 1987 Jun;11(2):147–153. doi: 10.1016/0165-0378(87)90018-0. [DOI] [PubMed] [Google Scholar]


