Abstract
Phytohaemagglutinin (PHA)-stimulated peripheral blood lymphocytes were examined sequentially for changes in volume, the appearance of cell membrane receptors and nucleic acid synthesis. The kinetics of appearance of activation antigens were compared with the progress of the cell through the separate events of volume growth and nucleic acid syntheses, to determine points at which regulation of receptors may control further progress through the cell cycle. In all samples tested there was a consistent pattern of response in the proportion of cells progressing through the cell cycle. Most of the T cells increased in size (mean 82% at 24 hr), fewer cells entered the Gla/Glb phase with the onset of RNA synthesis (mean 68% at 48 hr) and even fewer entered DNA synthesis (mean 42% at 72 hr). The time-course of appearance and the number of cells expressing IL-2 receptors were almost identical with that of cells responding by RNA synthesis. A similar correlation was observed between expression of the transferrin receptor and DNA synthesis. Addition of anti-Tac antibody temporarily suppressed the onset of RNA synthesis and antibodies to the transferrin receptor suppressed DNA synthesis. These linkages are further evidence that IL-2 and transferrin are the specific signals for cellular RNA and DNA synthesis. With optimal concentrations of PHA, addition of IL-2 did not increase the proportion of cells bearing activation antigens or undergoing nucleic acid synthesis. Suboptimal concentrations of PHA produced a small reduction in the number of cells expressing the IL-2 receptor, but a much greater reduction in the rate of entry into RNA synthesis. There was a consistent increase in all activation parameters tested with the addition of IL-2, but the proportion of cells expressing the transferrin receptor and entering DNA synthesis was consistently lower than that of cells that expressed the IL-2 receptor or entered RNA synthesis. This suggests that regulation of the IL-2 receptor is not responsible for the reduction in the number of cells that proceed to proliferation. The CD2 antigen (T11(1] showed increasing expression in a step-wise fashion after activation, the increases coinciding with the onset of RNA and DNA syntheses.
Full text
PDF

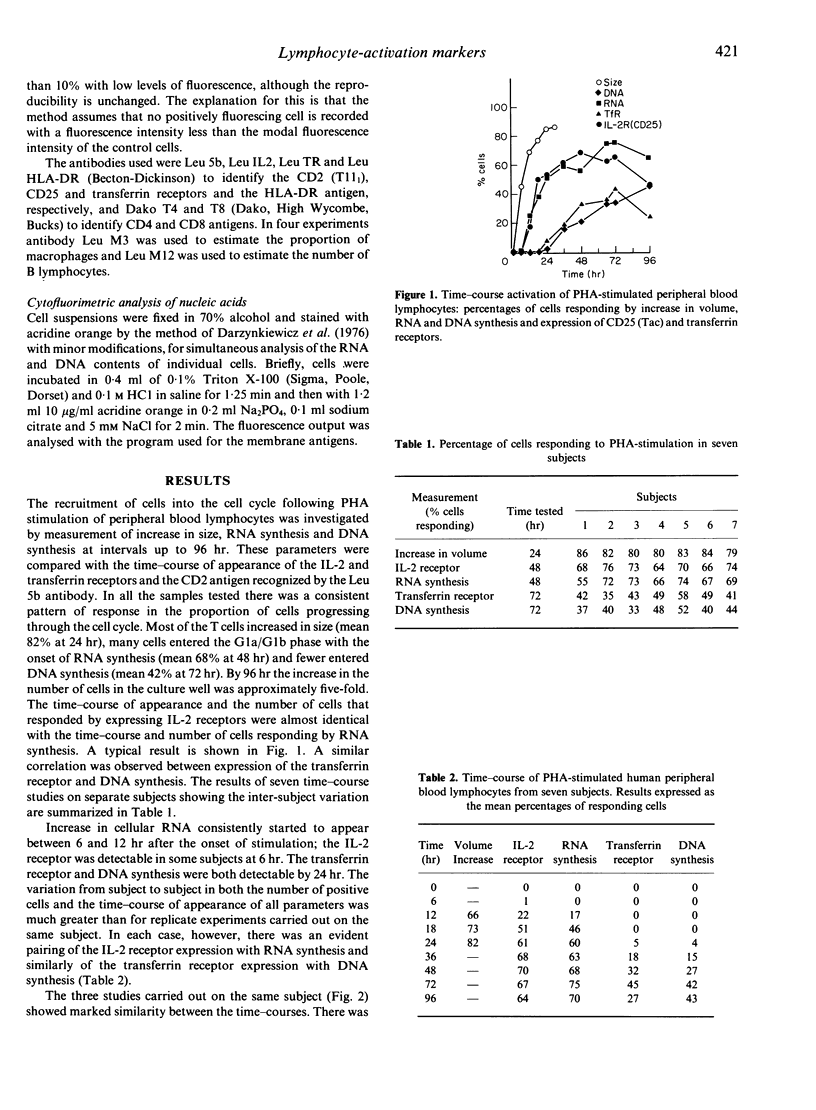
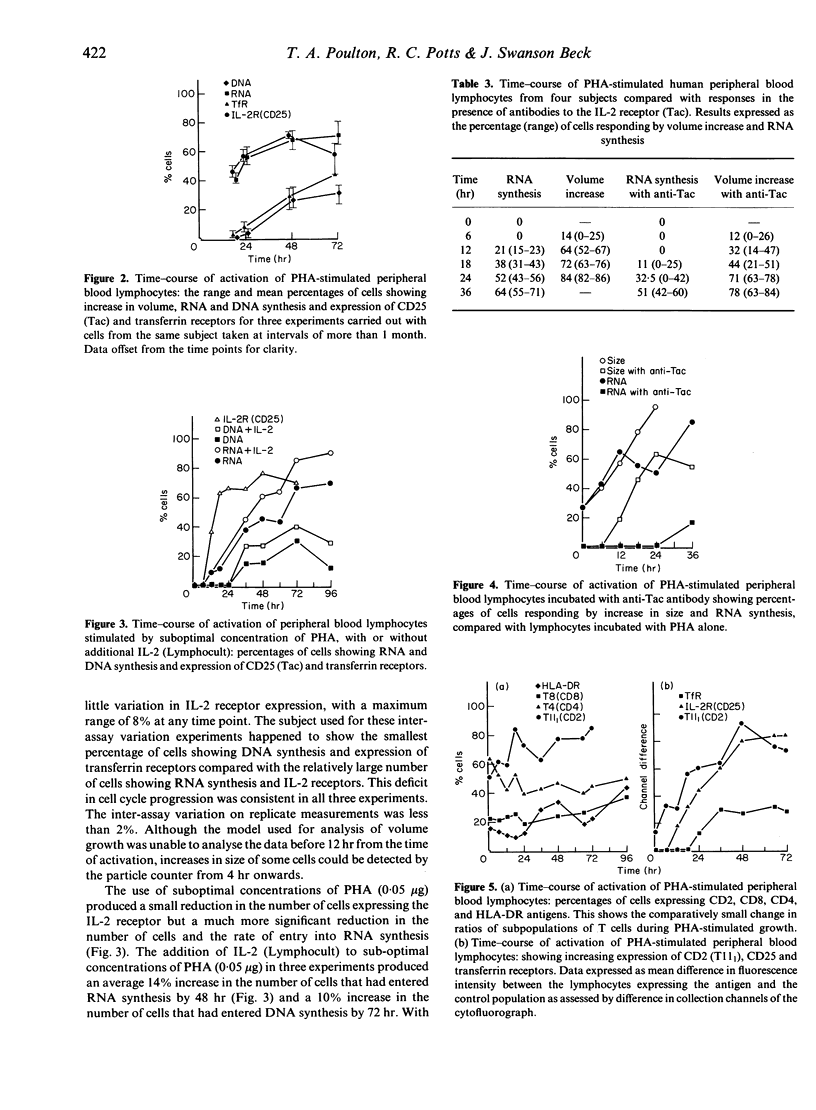



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettens F., Kristensen F., Walker C., Schwuléra U., Bonnard G. D., de Weck A. L. Lymphokine regulation of activated (G1) lymphocytes. II. Glucocorticoid and anti-Tac-induced inhibition of human T lymphocyte proliferation. J Immunol. 1984 Jan;132(1):261–265. [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers P. C. Determination of co-expression of activation antigens on proliferating CD4+, CD4+ CD8+ and CD8+ lymphocyte subsets by dual parameter flow cytometry. J Immunol Methods. 1987 Mar 12;97(2):165–171. doi: 10.1016/0022-1759(87)90456-x. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. H., Brown R. A., Robertson A. J., Potts R. C., Beck J. S. A new method of testing for mitogen-induced lymphocyte stimulation: measurement of the percentage of growing cells and of some aspects of their cell kinetics with an electronic particle counter. J Immunol Methods. 1979;25(2):147–158. doi: 10.1016/0022-1759(79)90050-4. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hussein Y. M., Kerr M. A., Beck J. S. The mechanism of action of the factor in leprosy serum that inhibits the growth of mitogen-stimulated normal human lymphocytes. Immunology. 1987 Jun;61(2):125–129. [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Isakov N., Bleackley R. C., Shaw J., Altman A. The tumor promoter teleocidin induces IL 2 receptor expression and IL 2-independent proliferation of human peripheral blood T cells. J Immunol. 1985 Oct;135(4):2343–2350. [PubMed] [Google Scholar]
- Kristensen F., Walker C., Joncourt F., Bettens F., de Weck A. L. Human lymphocyte proliferation. I. Correlation between activated and proliferating T-lymphocytes. Immunol Lett. 1982 Aug;5(2):59–63. doi: 10.1016/0165-2478(82)90033-5. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linch D. C., Wallace D. L., O'Flynn K. Signal transduction in human T lymphocytes. Immunol Rev. 1987 Feb;95:137–159. doi: 10.1111/j.1600-065x.1987.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Parks D. R., Rouse R. V., Herzenberg L. A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5147–5150. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Ashwell J. D. Interleukin 2 upregulates expression of its receptor on a T cell clone. J Exp Med. 1985 Jun 1;161(6):1575–1580. doi: 10.1084/jem.161.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet-Brown E. R., Lee J. W., Cheung R. K., Gelfand E. W. Antigen-specific and -nonspecific mitogenic signals in the activation of human T cell clones. J Immunol. 1987 Jun 1;138(11):3713–3719. [PubMed] [Google Scholar]
- Potts R. C., MacConnachie A., Brown R. A., Gibbs J. H., Robertson A. J., Hassan H. A., Beck J. S. Some tetracycline drugs suppress mitogen-stimulated lymphocyte growth but others do not. Br J Clin Pharmacol. 1983 Aug;16(2):127–132. doi: 10.1111/j.1365-2125.1983.tb04975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Sinclair W. K., Ross D. W. Modesl of growth in mammalian cells. Biophys J. 1969 Aug;9(8):1056–1070. doi: 10.1016/s0006-3495(69)86436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Andreeff M., Platzer E., Holloway K., Rubin B. Y., Moore M. A., Mertelsmann R. Interleukin 2 regulates the expression of Tac antigen on peripheral blood T lymphocytes. J Exp Med. 1984 Nov 1;160(5):1390–1403. doi: 10.1084/jem.160.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Loertscher R., Cotner T., Reddish M., Shapiro H. M., Carpenter C. B., Strominger J. L., Strom T. B. Dual parameter flow cytometric analysis of DNA content, activation antigen expression, and T cell subset proliferation in the human mixed lymphocyte reaction. J Immunol. 1984 May;132(5):2330–2337. [PubMed] [Google Scholar]
- Yagita H., Masuko T., Takahashi N., Hashimoto Y. Monoclonal antibodies that inhibit activation and proliferation of lymphocytes. I. Expression of the antigen on monocytes and activated lymphocytes. J Immunol. 1986 Mar 15;136(6):2055–2061. [PubMed] [Google Scholar]


