Abstract
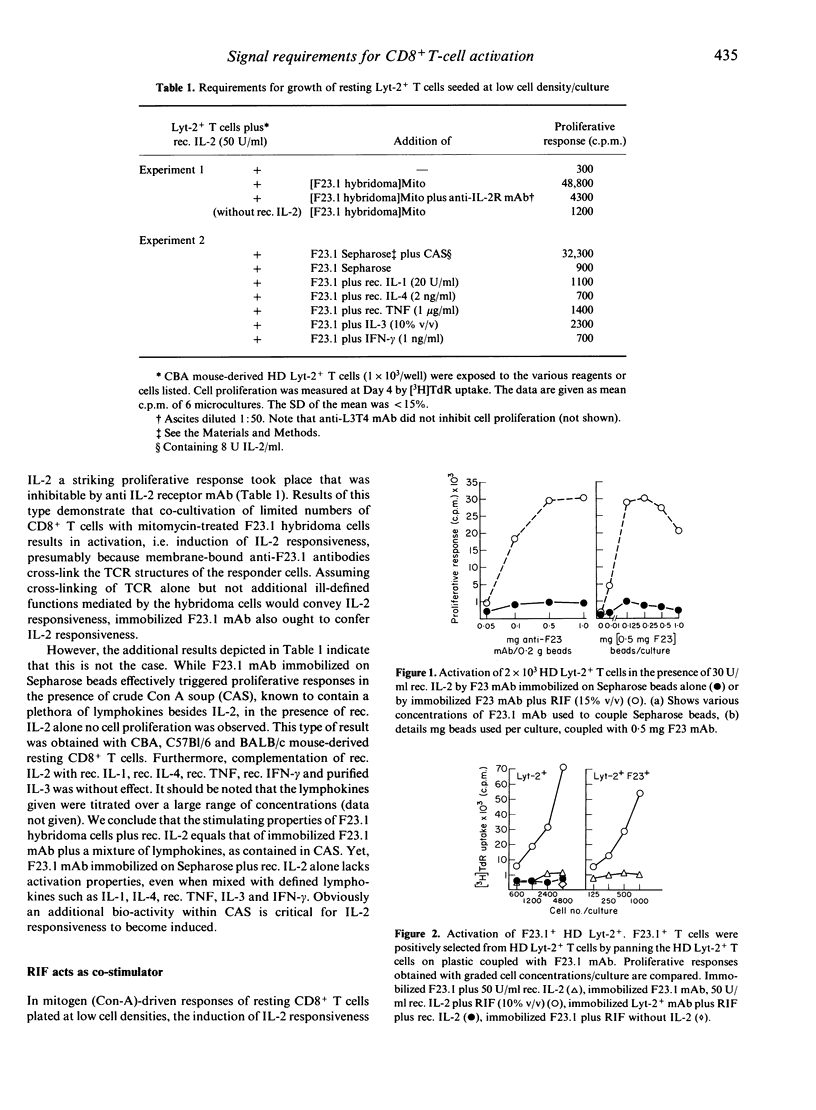
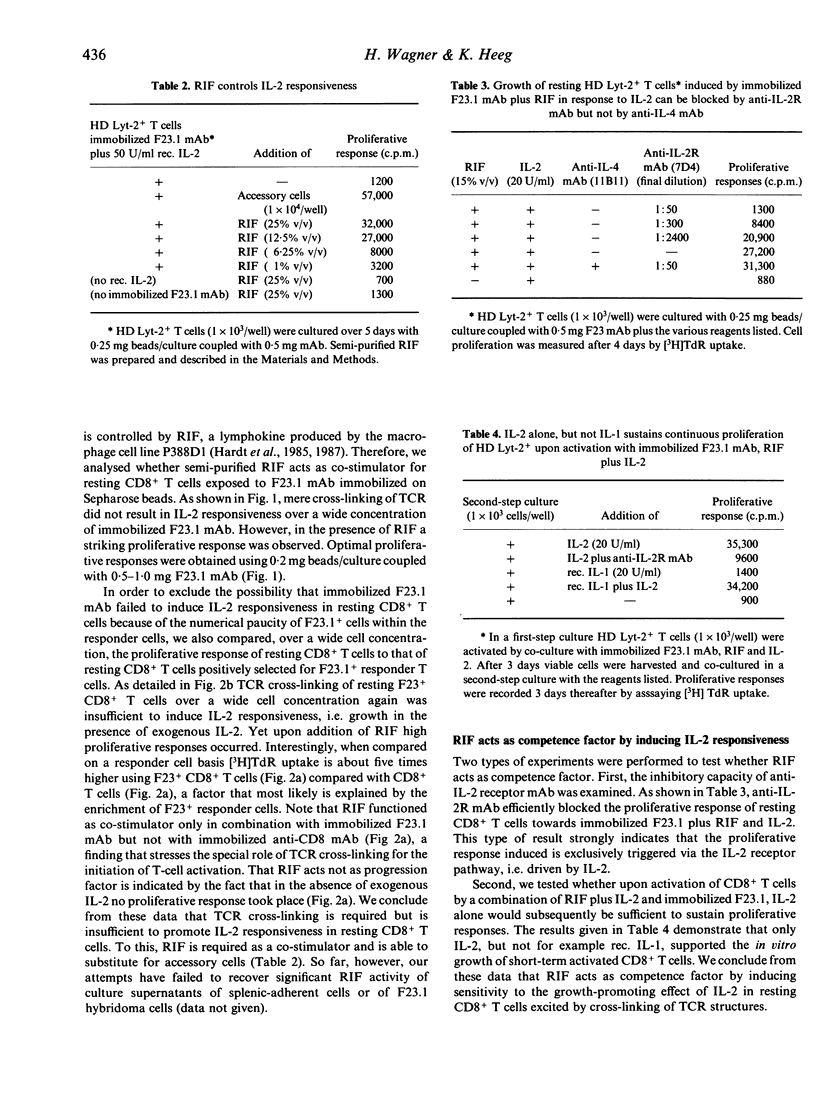
In the model system used here, cross-linking of T-cell receptor structures (TCR) by antigen-presenting cells (APCs) is substituted by the use of anti-F23.1 anti-T-cell receptor monoclonal antibody immobilized on Sepharose beads. We show that CR cross-linking of resting murine CD8+ T cells seeded at low cell densities is insufficient to induce responsiveness to the growth-promoting effect of interleukin-2 (IL-2), i.e. fails to induce expression of functional IL-2 receptors. The macrophage cell-line product, IL-2 receptor-inducing factor (RIF), but not IL-1, IL-3, IL-4 and interferon-gamma (IFN-gamma) functions efficiently as a co-stimulator. Once activated, growth of CD8+ T cells is driven entirely by IL-2. We conclude that two restriction points control the activation of resting CD8+ T cells. While cross-linking of TCR is essential as the first step, RIF is required as the competence factor to induce IL-2 responsiveness. We consider the possibility that the ability of APCs to produce RIF determines the immunogenicity of APCs towards antigen-reactive resting CD8+ T cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eichmann K., Jönsson J. I., Falk I., Emmrich F. Effective activation of resting mouse T lymphocytes by cross-linking submitogenic concentrations of the T cell antigen receptor with either Lyt-2 or L3T4. Eur J Immunol. 1987 May;17(5):643–650. doi: 10.1002/eji.1830170510. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erard F., Corthesy P., Nabholz M., Lowenthal J. W., Zaech P., Plaetinck G., MacDonald H. R. Interleukin 2 is both necessary and sufficient for the growth and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Immunol. 1985 Mar;134(3):1644–1652. [PubMed] [Google Scholar]
- Erard F., Nabholz M., MacDonald H. R. Antigen stimulation of cytolytic T lymphocyte precursors: minimal requirements for growth and acquisition of cytolytic activity. Eur J Immunol. 1985 Aug;15(8):798–803. doi: 10.1002/eji.1830150811. [DOI] [PubMed] [Google Scholar]
- Hardt C. Activation of murine CD8+ lymphocytes: two distinct signals regulate c-myc and interleukin 2 receptor RNA expression. Eur J Immunol. 1987 Dec;17(12):1711–1717. doi: 10.1002/eji.1830171206. [DOI] [PubMed] [Google Scholar]
- Hardt C., Diamantstein T., Wagner H. Signal requirements for the in vitro differentiation of cytotoxic T lymphocytes (CTL): distinct soluble mediators promote preactivation of CTL-precursors, clonal growth and differentiation into cytotoxic effector cells. Eur J Immunol. 1985 May;15(5):472–478. doi: 10.1002/eji.1830150511. [DOI] [PubMed] [Google Scholar]
- Hardt C., Sato N., Wagner H. Functional and biochemical characteristics of a murine interleukin 2 receptor-inducing factor. Eur J Immunol. 1987 Feb;17(2):209–216. doi: 10.1002/eji.1830170210. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Andrus L., Prowse S. J. Role of lymphokine and antigen in the control of specific T cell responses. Immunol Rev. 1980;51:279–314. doi: 10.1111/j.1600-065x.1980.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Gullberg M., Bandeira A., Coutinho A. Activation and growth requirements for cytotoxic and noncytotoxic T lymphocytes. Cell Immunol. 1984 Nov;89(1):223–231. doi: 10.1016/0008-8749(84)90212-0. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Wallace D. L., O'Flynn K. Signal transduction in human T lymphocytes. Immunol Rev. 1987 Feb;95:137–159. doi: 10.1111/j.1600-065x.1987.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Meyer zum Büschenfelde K. H. T cell receptor triggering induces responsiveness to interleukin 1 and interleukin 2 but does not lead to T cell proliferation. J Immunol. 1986 Jun 1;136(11):4106–4112. [PubMed] [Google Scholar]
- Nabholz M., MacDonald H. R. Cytolytic T lymphocytes. Annu Rev Immunol. 1983;1:273–306. doi: 10.1146/annurev.iy.01.040183.001421. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Zanders E. D., Lamb J. R., Beverley P. C., Wallace D. L., Tatham P. E., Tax W. J., Linch D. C. Investigation of early T cell activation: analysis of the effect of specific antigen, interleukin 2 and monoclonal antibodies on intracellular free calcium concentration. Eur J Immunol. 1985 Jan;15(1):7–11. doi: 10.1002/eji.1830150103. [DOI] [PubMed] [Google Scholar]
- Palacios R. Mechanisms by which accessory cells contribute in growth of resting T lymphocytes initiated by OKT3 antibody. Eur J Immunol. 1985 Jul;15(7):645–651. doi: 10.1002/eji.1830150702. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Scheurich P., Däubener W., Krönke M., Röllinghoff M., Wagner H. Quantitative representation of all T cells committed to develop into cytotoxic effector cells and/or interleukin 2 activity-producing helper cells within murine T lymphocyte subsets. Eur J Immunol. 1984 Jan;14(1):33–39. doi: 10.1002/eji.1830140107. [DOI] [PubMed] [Google Scholar]
- Schmidberger R., Miethke T., Heeg K., Wagner H. Primary activation of murine CD8 T cells via cross-linking of T3 cell surface structures: two signals regulate induction of interleukin 2 responsiveness. Eur J Immunol. 1988 Feb;18(2):277–282. doi: 10.1002/eji.1830180215. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M. V., Pasternack M. S., Lugo J. P., Klein J. R., Eisen H. N. Isolation and partial characterization of concanavalin A receptors on cloned cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1519–1523. doi: 10.1073/pnas.81.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerz U. D., Bevan M. J. Activation of resting T lymphocytes by a monoclonal antibody directed against an allotypic determinant on the T cell receptor. Eur J Immunol. 1986 Mar;16(3):263–270. doi: 10.1002/eji.1830160310. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohr H. W., Hünig T. Induction of proliferative and cytotoxic responses in resting Lyt-2+ T cells with lectin and recombinant interleukin 2. Eur J Immunol. 1985 Apr;15(4):332–337. doi: 10.1002/eji.1830150405. [DOI] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Pfizenmaier K., Solbach W., Bartlett R., Stockinger H., Röllinghoff M. T-T cell interactions during cytotoxic T lymphocyte (CTL) responses: T cell derived helper factor (Interleukin 2) as a probe to analyze CTL responsiveness and thymic maturation of CTL progenitors. Immunol Rev. 1980;51:215–255. doi: 10.1111/j.1600-065x.1980.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Widmer M. B., Grabstein K. H. Regulation of cytolytic T-lymphocyte generation by B-cell stimulatory factor. Nature. 1987 Apr 23;326(6115):795–798. doi: 10.1038/326795a0. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


