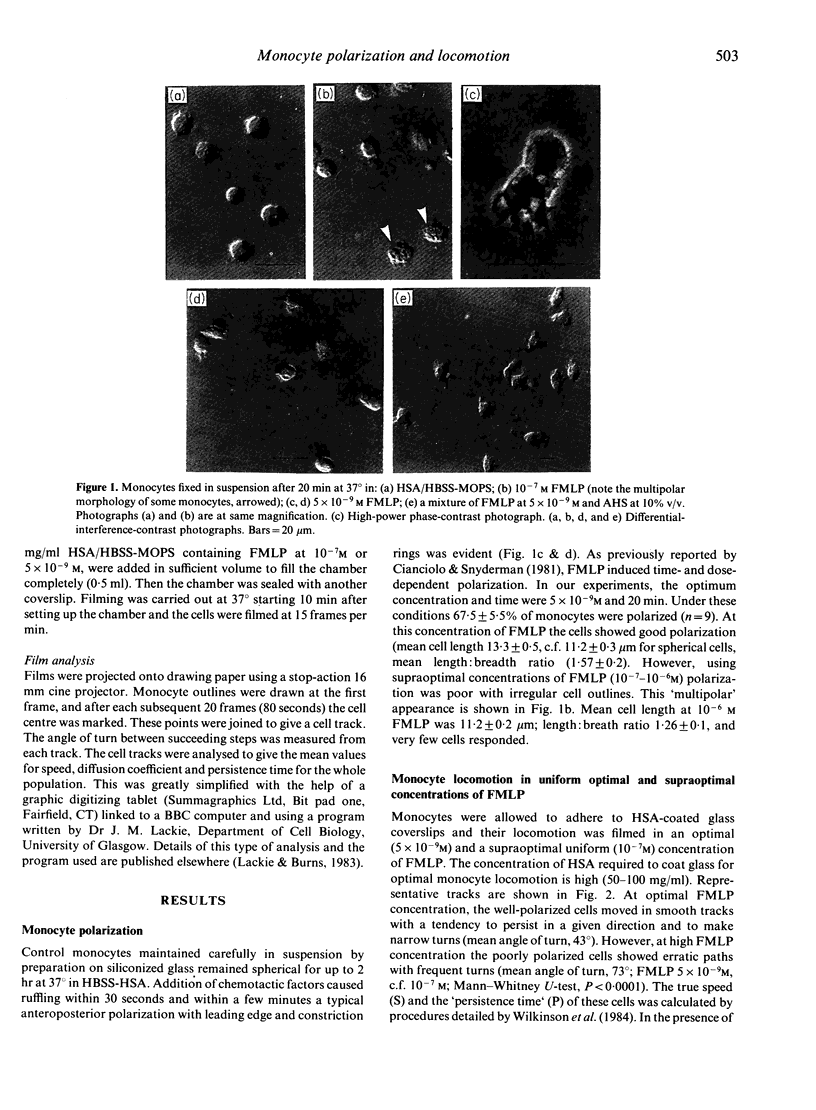
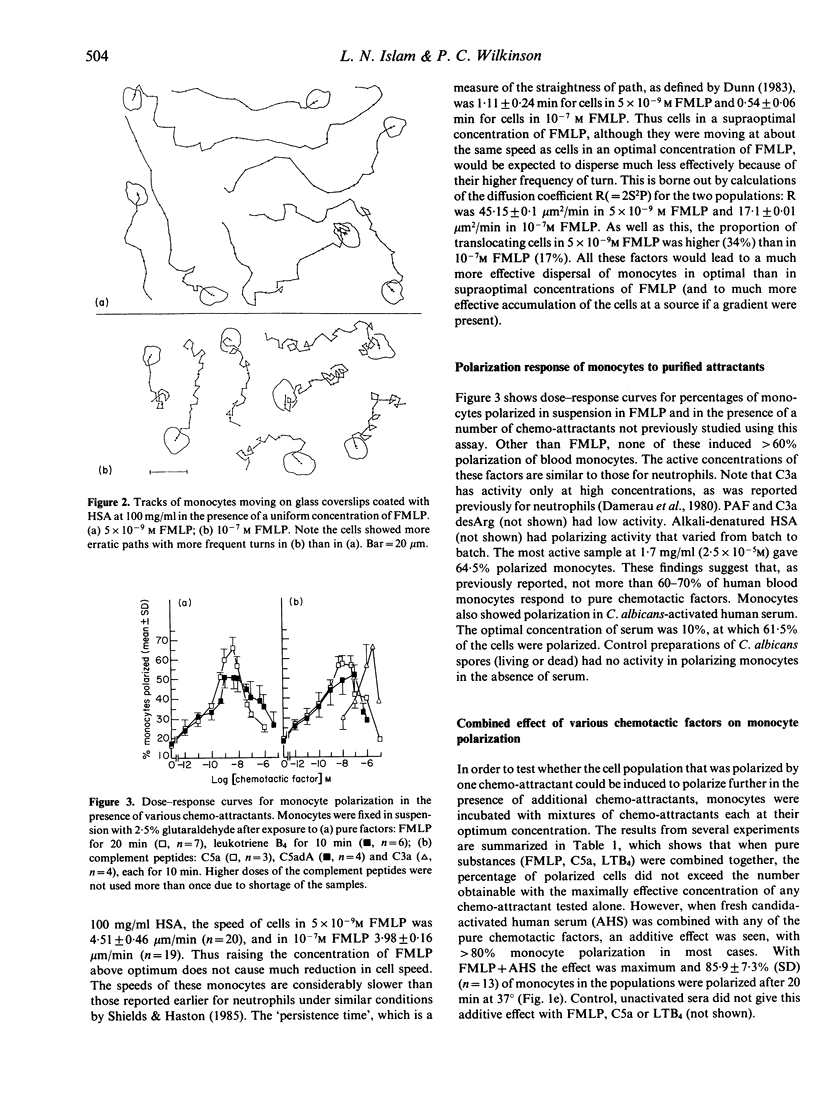
Abstract
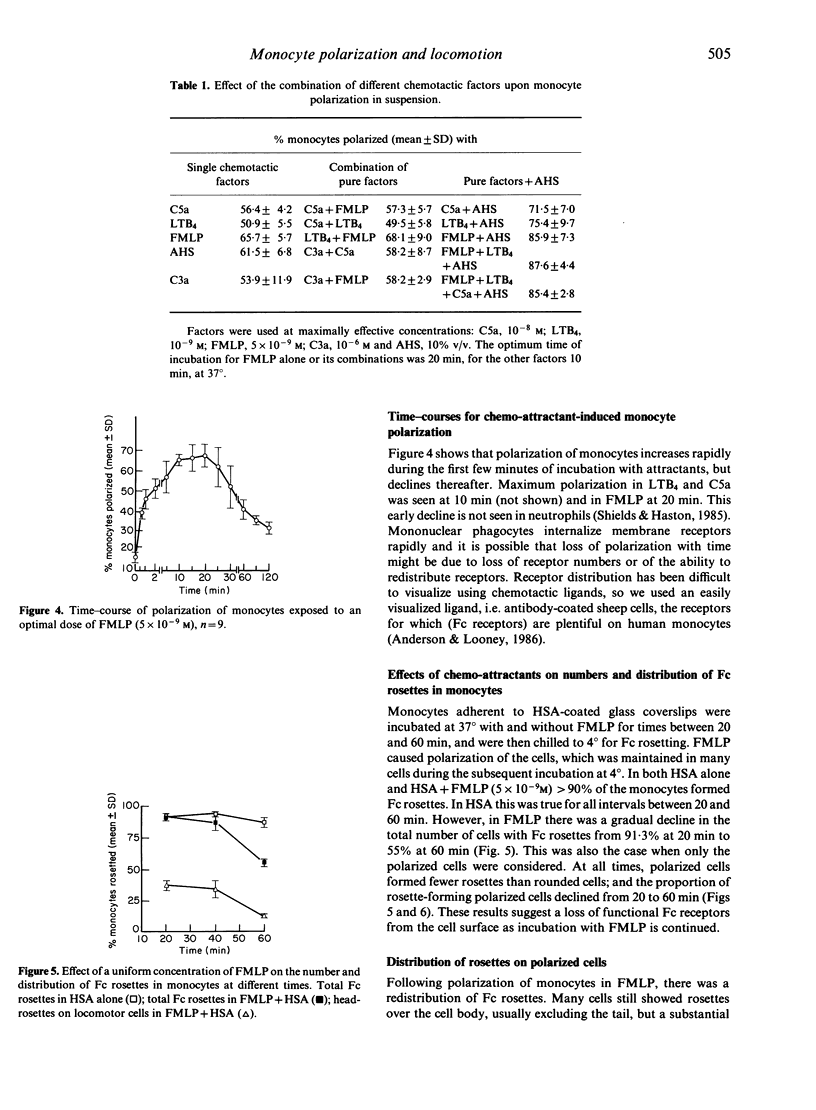
The locomotor response of human blood monocytes to chemotactic factors was studied using a polarization assay on cells in suspension and by filming locomotion on albumin-coated glass. Cells in optimal (5 x 10(-9) M) but uniform concentrations of N-formyl-methionyl-leucyl-phenylalanine (FMLP) polarized well and showed a 'persistent random walk' type of locomotion, whereas in supraoptimal concentrations (10(-7) M), the cells took erratic paths and polarized poorly, suggesting that monocytes cannot develop an anteroposterior polarity if hit by ligand molecules at many points on the cell surface simultaneously. Monocyte polarization in chemotactic factors at 37 degrees was transient and was gradually lost after 15-20 min. Likewise, the ability to form Fc rosettes after this time was gradually lost, suggesting loss of functional receptors from the cell surface with time. In optimally polarized cells, Fc rosettes were frequently localized at the head of the cell. This localization also was lost with time. Using pure chemotactic factors (FMLP, C5a, leukotriene B4) we found, as reported earlier (Cianciolo & Snyderman 1981), that polarization was restricted to a subpopulation (approximately 60% of cells) that responded to multiple attractants. However, 80-90% of monocytes polarized in response to combinations of any of the above pure attractants with candida-activated serum. This suggests that the subpopulation that lacks receptors for classical chemotactic factors nevertheless has locomotor capacity and can respond to undefined factors in activated serum, and that the great majority of blood monocytes is motile if appropriately stimulated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Allan R. B., Wilkinson P. C. A visual analysis of chemotactic and chemokinetic locomotion of human neutrophil leucocytes. Use of a new chemotaxis assay with Candida albicans as gradient source. Exp Cell Res. 1978 Jan;111(1):191–203. doi: 10.1016/0014-4827(78)90249-5. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerau B., Zimmermann B., Grünefeld E., Czorniak K., Vogt W. Biological activities of C5a and C5adesArg from hog serum. Int Arch Allergy Appl Immunol. 1980;63(4):408–414. doi: 10.1159/000232656. [DOI] [PubMed] [Google Scholar]
- Detmers P. A., Wright S. D., Olsen E., Kimball B., Cohn Z. A. Aggregation of complement receptors on human neutrophils in the absence of ligand. J Cell Biol. 1987 Sep;105(3):1137–1145. doi: 10.1083/jcb.105.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. A. Characterising a kinesis response: time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 1983;12:14–33. doi: 10.1007/978-3-0348-9352-7_1. [DOI] [PubMed] [Google Scholar]
- Falk W., Leonard E. J. Human monocyte chemotaxis: migrating cells are a subpopulation with multiple chemotaxin specificities on each cell. Infect Immun. 1980 Sep;29(3):953–959. doi: 10.1128/iai.29.3.953-959.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Haston W. S., Shields J. M. Neutrophil leucocyte chemotaxis: a simplified assay for measuring polarizing responses to chemotactic factors. J Immunol Methods. 1985 Aug 2;81(2):229–237. doi: 10.1016/0022-1759(85)90208-x. [DOI] [PubMed] [Google Scholar]
- Haston W. S., Wilkinson P. C. Gradient perception by neutrophil leucocytes. J Cell Sci. 1987 Apr;87(Pt 3):373–374. doi: 10.1242/jcs.87.3.373. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Zimmermann A., Cottier H. Crawling-like movements, adhesion to solid substrata and chemokinesis of neutrophil granulocytes. J Cell Sci. 1983 Nov;64:89–106. doi: 10.1242/jcs.64.1.89. [DOI] [PubMed] [Google Scholar]
- Lackie J. M., Burns M. D. Leucocyte locomotion: comparison of random and directed paths using a modified time-lapse film analysis. J Immunol Methods. 1983 Aug 12;62(1):109–122. doi: 10.1016/0022-1759(83)90116-3. [DOI] [PubMed] [Google Scholar]
- Ohura K., Katona I. M., Wahl L. M., Chenoweth D. E., Wahl S. M. Co-expression of chemotactic ligand receptors on human peripheral blood monocytes. J Immunol. 1987 Apr 15;138(8):2633–2639. [PubMed] [Google Scholar]
- Pike M. C., Fischer D. G., Koren H. S., Snyderman R. Development of specific receptors for N-formylated chemotactic peptides in a human monocyte cell line stimulated with lymphokines. J Exp Med. 1980 Jul 1;152(1):31–40. doi: 10.1084/jem.152.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields J. M., Haston W. S. Behaviour of neutrophil leucocytes in uniform concentrations of chemotactic factors: contraction waves, cell polarity and persistence. J Cell Sci. 1985 Mar;74:75–93. doi: 10.1242/jcs.74.1.75. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. Motility and adhesiveness in human neutrophils. Redistribution of chemotactic factor-induced adhesion sites. J Clin Invest. 1980 Apr;65(4):804–812. doi: 10.1172/JCI109731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R. J., Berlin R. D., Oliver J. M. Asymmetric Fc receptor distribution on human PMN oriented in a chemotactic gradient. Nature. 1980 Aug 14;286(5774):724–725. doi: 10.1038/286724a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Bradley G. R. Chemotactic and enzyme-releasing activity of amphipathic proteins for neutrophils. A possible role for protease in chemotaxis on substratum-bound protein gradients. Immunology. 1981 Apr;42(4):637–648. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C., Lackie J. M., Forrester J. V., Dunn G. A. Chemokinetic accumulation of human neutrophils on immune complex-coated substrata: analysis at a boundary. J Cell Biol. 1984 Nov;99(5):1761–1768. doi: 10.1083/jcb.99.5.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. The locomotor capacity of human lymphocytes and its enhancement by cell growth. Immunology. 1986 Feb;57(2):281–289. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Visual observations of chemotaxis and chemotropism in mouse macrophages. Immunobiology. 1982 Apr;161(3-4):376–384. doi: 10.1016/S0171-2985(82)80095-8. [DOI] [PubMed] [Google Scholar]
- van Epps D. E., Chenoweth D. E. Analysis of the binding of fluorescent C5a and C3a to human peripheral blood leukocytes. J Immunol. 1984 Jun;132(6):2862–2867. [PubMed] [Google Scholar]