Abstract
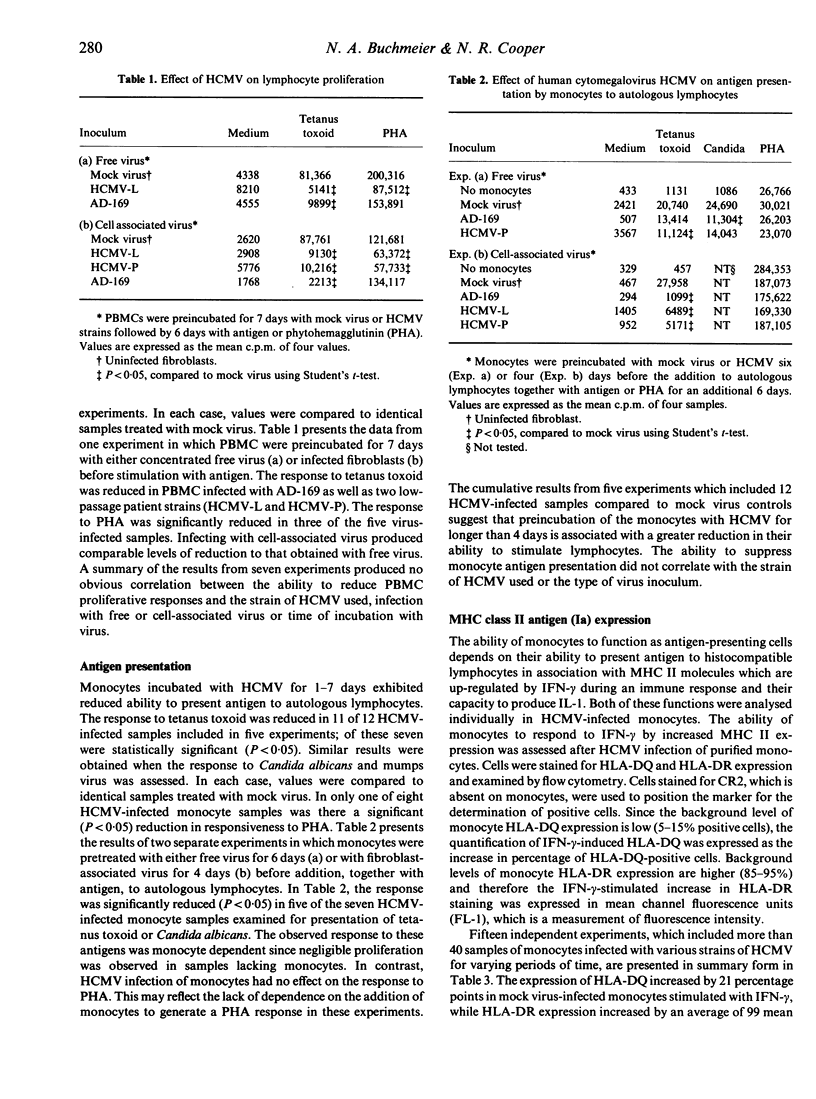
The role of the monocyte in human cytomegalovirus (HCMV)-induced immunosuppression was examined by assessing the ability of the virus to directly suppress various monocyte accessory cell functions. Both patient-derived and laboratory-adapted strains of HCMV were capable of impairing antigen-presenting functions of purified human monocytes. In seven of 12 virus-infected samples, there was a significant decrease (P less than 0.05) in the ability of HCMV-infected monocytes to present tetanus toxoid to autologous lymphocytes compared with mock-infected controls; similar results were obtained with Candida albicans and mumps. In contrast, the response to PHA was impaired in only one of eight HCMV-infected samples. The increased expression of MHC class II Ia antigens (HLA-DQ and HLA-DR) by monocytes after stimulation by interferon-gamma was impaired in approximately one-third of the 43 virus-infected samples tested. Interleukin-1 (IL-1) production after incubation with the stimulating antigens, however, was unaffected. Attempts to augment immuno-suppression by co-stimulation of monocytes with lipopolysaccharide (LPS), heat-killed Escherichia coli or Listeria monocytogenes were not successful; however, dramatically increased levels of immunosuppression was obtained with HCMV preparations containing mycoplasma. Thus, although HCMV is capable of directly perturbing monocyte accessory cell functions, the variability and partial suppression observed suggests that infection of monocytes by HCMV alone is not sufficient to produce the levels of immune hyporesponsiveness observed in HCMV-infected patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carney W. P., Hirsch M. S. Mechanisms of immunosuppression in cytomegalovirus mononucleosis. II. Virus-monocyte interactions. J Infect Dis. 1981 Jul;144(1):47–54. doi: 10.1093/infdis/144.1.47. [DOI] [PubMed] [Google Scholar]
- Darai G., Flügel R. M., Zöller L., Matz B., Kreig A., Gelderblom H., Delius H., Leach R. H. The plaque-forming factor for mink lung cells present in cytomegalovirus and herpes-zoster virus stocks identified as Mycoplasma hyorhinis. J Gen Virol. 1981 Jul;55(Pt 1):201–205. doi: 10.1099/0022-1317-55-1-201. [DOI] [PubMed] [Google Scholar]
- Dudding L. R., Garnett H. M. Interaction of strain AD169 and a clinical isolate of cytomegalovirus with peripheral monocytes: the effect of lipopolysaccharide stimulation. J Infect Dis. 1987 May;155(5):891–896. doi: 10.1093/infdis/155.5.891. [DOI] [PubMed] [Google Scholar]
- Einhorn L., Ost A. Cytomegalovirus infection of human blood cells. J Infect Dis. 1984 Feb;149(2):207–214. doi: 10.1093/infdis/149.2.207. [DOI] [PubMed] [Google Scholar]
- Esparza I., Fox R. I., Schreiber R. D. Interferon-gamma-dependent modulation of C3b receptors (CR1) on human peripheral blood monocytes. J Immunol. 1986 Feb 15;136(4):1360–1365. [PubMed] [Google Scholar]
- Jacobs D. B., Pipho C. Use of propidium iodide staining and flow cytometry to measure anti-mediated cytotoxicity: resolution of complement-sensitive and resistant target cells. J Immunol Methods. 1983 Aug 12;62(1):101–108. doi: 10.1016/0022-1759(83)90115-1. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Levin M. J., Rinaldo C. R., Jr, Leary P. L., Zaia J. A., Hirsch M. S. Immune response to herpesvirus antigens in adults with acute cytomegaloviral mononucleosis. J Infect Dis. 1979 Dec;140(6):851–857. doi: 10.1093/infdis/140.6.851. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- RUEBNER B. H., HIRANO T., SLUSSER R. J., MEDEARIS D. N., Jr HUMAN CYTOMEGALOVIRUS INFECTION. ELECTRON MICROSCOPIC AND HISTOCHEMICAL CHANGES IN CULTURES OF HUMAN FIBROBLASTS. Am J Pathol. 1965 Mar;46:477–496. [PMC free article] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Carney W. P., Richter B. S., Black P. H., Hirsch M. S. Mechanisms of immunosuppression in cytomegaloviral mononucleosis. J Infect Dis. 1980 Apr;141(4):488–495. doi: 10.1093/infdis/141.4.488. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Oldstone M. B. Recent clinical isolates of cytomegalovirus suppress human cytomegalovirus-specific human leukocyte antigen-restricted cytotoxic T-lymphocyte activity. J Virol. 1986 Jul;59(1):127–131. doi: 10.1128/jvi.59.1.127-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Rice G. P., Oldstone M. B. Suppression of natural killer cell activity and T cell proliferation by fresh isolates of human cytomegalovirus. J Infect Dis. 1986 Jun;153(6):1084–1091. doi: 10.1093/infdis/153.6.1084. [DOI] [PubMed] [Google Scholar]
- Shenton B. K., Proud G., Smith B. M., Taylor R. M. Identification of immunosuppressive factors in plasma following multiple blood transfusions. Transplant Proc. 1979 Mar;11(1):171–174. [PubMed] [Google Scholar]
- Simberkoff M. S., Thorbecke G. J., Thomas L. Studies of PPLO infection. V. Inhibition of lymphocyte mitosis and antibody formation by mycoplasmal extracts. J Exp Med. 1969 Jun 1;129(6):1163–1181. doi: 10.1084/jem.129.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiku K., Tiku M. L., Liu S., Skosey J. L. Normal human neutrophils are a source of a specific interleukin 1 inhibitor. J Immunol. 1986 May 15;136(10):3686–3692. [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Wahren B., Ljungman P., Paulin T., Ringdén O. Enhancive and suppressive effects of cytomegalovirus on human lymphocyte responses in vitro. J Virol. 1986 Jun;58(3):909–913. doi: 10.1128/jvi.58.3.909-913.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


