Abstract
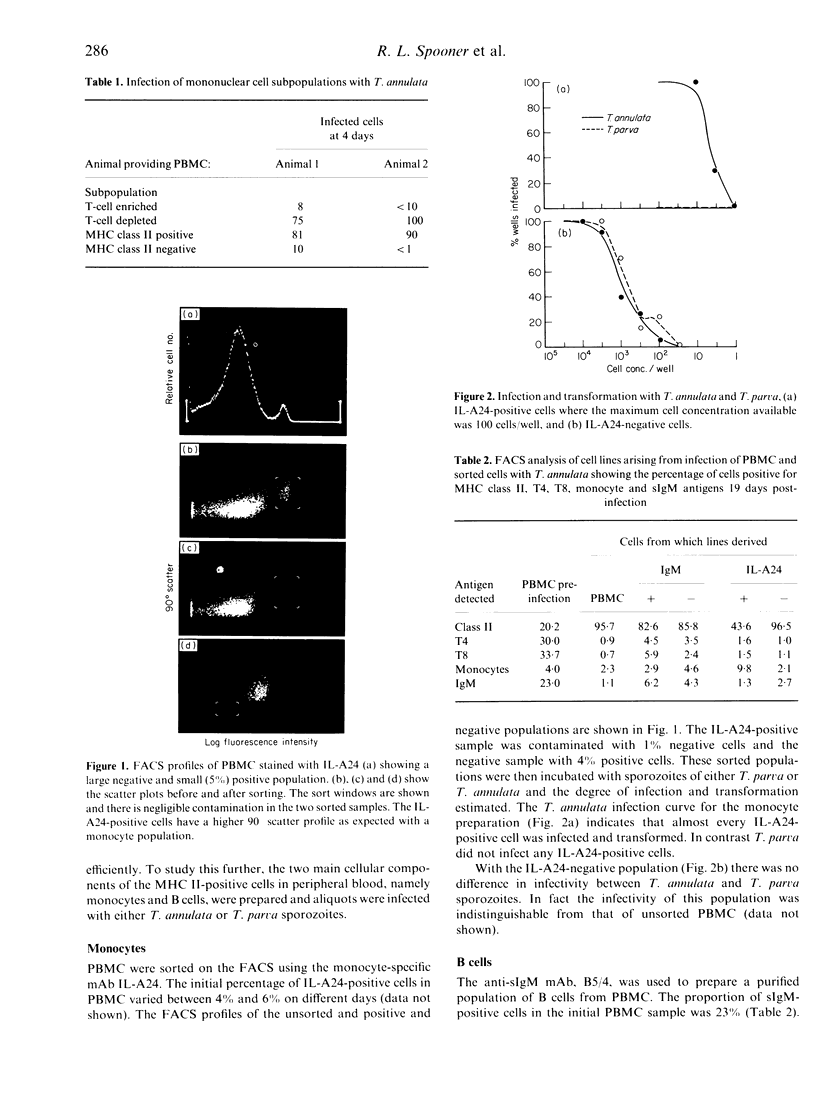
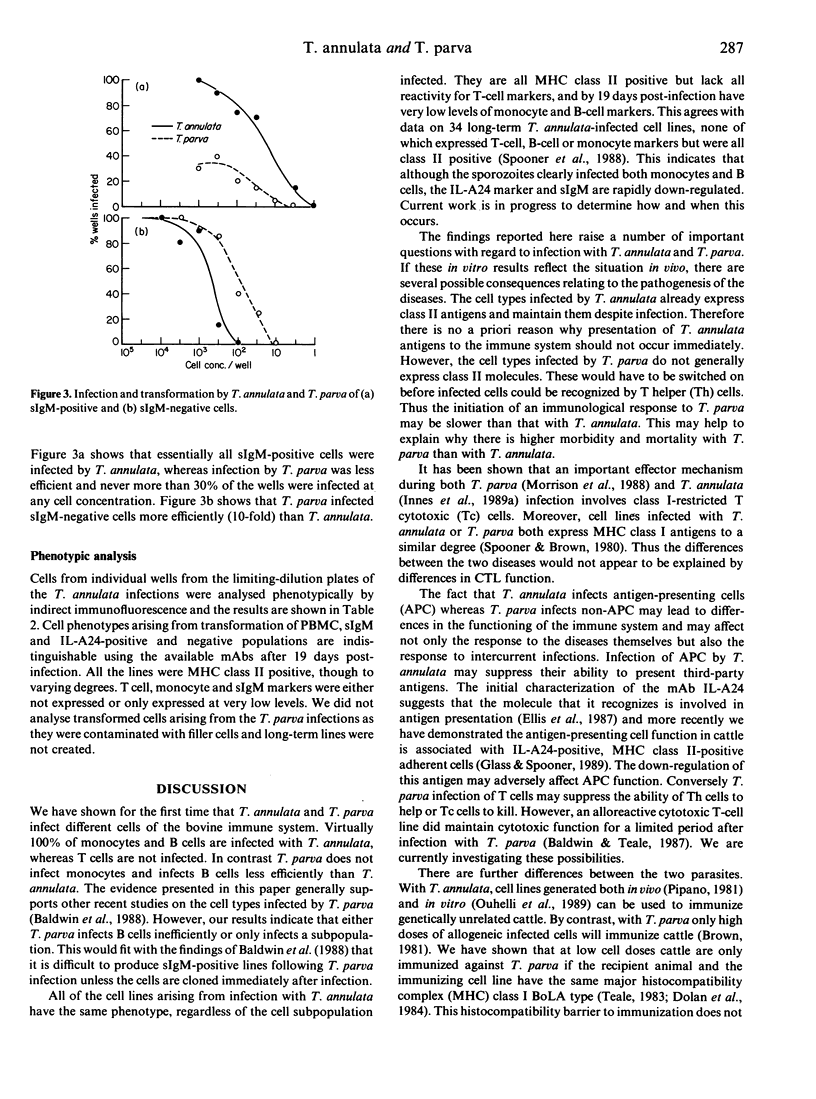
Bovine peripheral blood mononuclear cells (PBMC) were labelled with monoclonal antibodies recognizing bovine MHC class II, sIgM, monocyte, T-helper and T-cytotoxic cell phenotypes. They were sorted into positive and negative populations with a fluorescence-activated cell sorter (FACS). The cell populations were infected in vitro with sporozoites of either Theileria annulata or T. parva, and the degree of infection and transformation determined. The results showed that despite the many similarities between these two parasites, they infected different cells of the immune system. T. annulata preferentially infected MHC class II-positive cells but did not infect T cells. Monocytes were infected very efficiently by T. annulata but were uninfectable with T. parva. B cells were infected much more efficiently by T. annulata than T. parva. Cell lines derived from infections with T. annulata were analysed phenotypically. Virtually all reactivity was lost for the anti-sIgM and the anti-monocyte monoclonal antibodies post-infection and no T-cell markers were detected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. L., Black S. J., Brown W. C., Conrad P. A., Goddeeris B. M., Kinuthia S. W., Lalor P. A., MacHugh N. D., Morrison W. I., Morzaria S. P. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect Immun. 1988 Feb;56(2):462–467. doi: 10.1128/iai.56.2.462-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. L., Teale A. J. Alloreactive T cell clones transformed by Theileria parva retain cytolytic activity and antigen specificity. Eur J Immunol. 1987 Dec;17(12):1859–1862. doi: 10.1002/eji.1830171230. [DOI] [PubMed] [Google Scholar]
- Baldwin C. L., Teale A. J., Naessens J. G., Goddeeris B. M., MacHugh N. D., Morrison W. I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986 Jun 15;136(12):4385–4391. [PubMed] [Google Scholar]
- Dolan T. T., Teale A. J., Stagg D. A., Kemp S. J., Cowan K. M., Young A. S., Groocock C. M., Leitch B. L., Spooner R. L., Brown C. G. A histocompatibility barrier to immunization against East Coast fever using Theileria parva-infected lymphoblastoid cell lines. Parasite Immunol. 1984 May;6(3):243–250. doi: 10.1111/j.1365-3024.1984.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., Morrison W. I., Goddeeris B. M., Emery D. L. Bovine mononuclear phagocytic cells: identification by monoclonal antibodies and analysis of functional properties. Vet Immunol Immunopathol. 1987 Dec;17(1-4):125–134. doi: 10.1016/0165-2427(87)90133-4. [DOI] [PubMed] [Google Scholar]
- Moulton J., Buscher G., Bovell D., Doxsey S. Blast transformation of adherent macrophages infected in vitro with sporozoites of Theileria parva. Am J Vet Res. 1984 Apr;45(4):678–684. [PubMed] [Google Scholar]
- Naessens J., Newson J., Bensaid A., Teale A. J., Magondu J. G., Black S. J. De novo expression of T cell markers on Theileria parva-transformed lymphoblasts in cattle. J Immunol. 1985 Dec;135(6):4183–4188. [PubMed] [Google Scholar]
- Pinder M., Musoke A. J., Morrison W. I., Roelants G. E. The bovine lymphoid system. III. A monoclonal antibody specific for bovine cell surface and serum IgM. Immunology. 1980 Jul;40(3):359–365. [PMC free article] [PubMed] [Google Scholar]
- Spooner R. L., Brown C. G. Bovine lymphocyte antigens (BoLA) of bovine lymphocytes and derived lymphoblastoid lines transformed by Theileria parva and Theileria annulata. Parasite Immunol. 1980 Autumn;2(3):163–174. doi: 10.1111/j.1365-3024.1980.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Spooner R. L., Innes E. A., Glass E. J., Millar P., Brown C. G. Bovine mononuclear cell lines transformed by Theileria parva or Theileria annulata express different subpopulation markers. Parasite Immunol. 1988 Nov;10(6):619–629. doi: 10.1111/j.1365-3024.1988.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Teale A. J., Baldwin C. L., Ellis J. A., Newson J., Goddeeris B. M., Morrison W. I. Alloreactive bovine T lymphocyte clones: an analysis of function, phenotype, and specificity. J Immunol. 1986 Jun 15;136(12):4392–4398. [PubMed] [Google Scholar]
- Walker A. R., Fletcher J. D., McKellar S. B., Bell L. J., Brown C. G. The maintenance and survival of Theileria annulata in colonies of Hyalomma anatolicum anatolicum. Ann Trop Med Parasitol. 1985 Apr;79(2):199–209. doi: 10.1080/00034983.1985.11811907. [DOI] [PubMed] [Google Scholar]


