Abstract
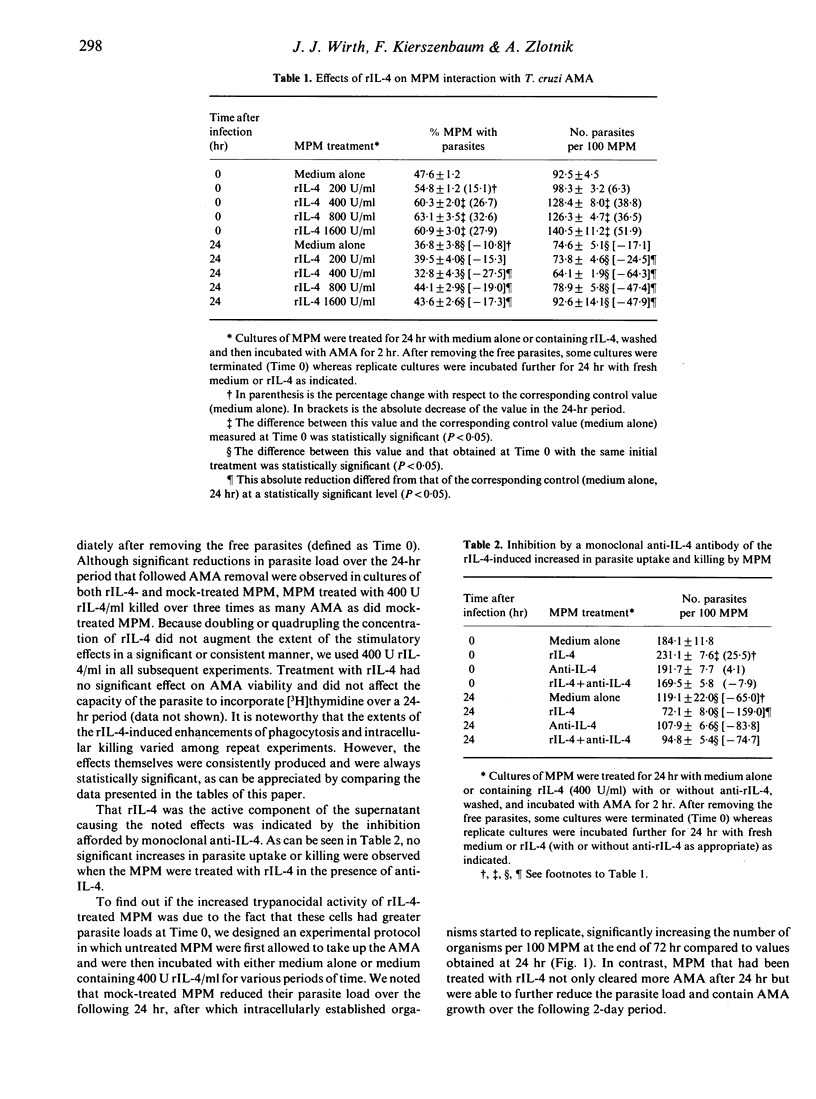
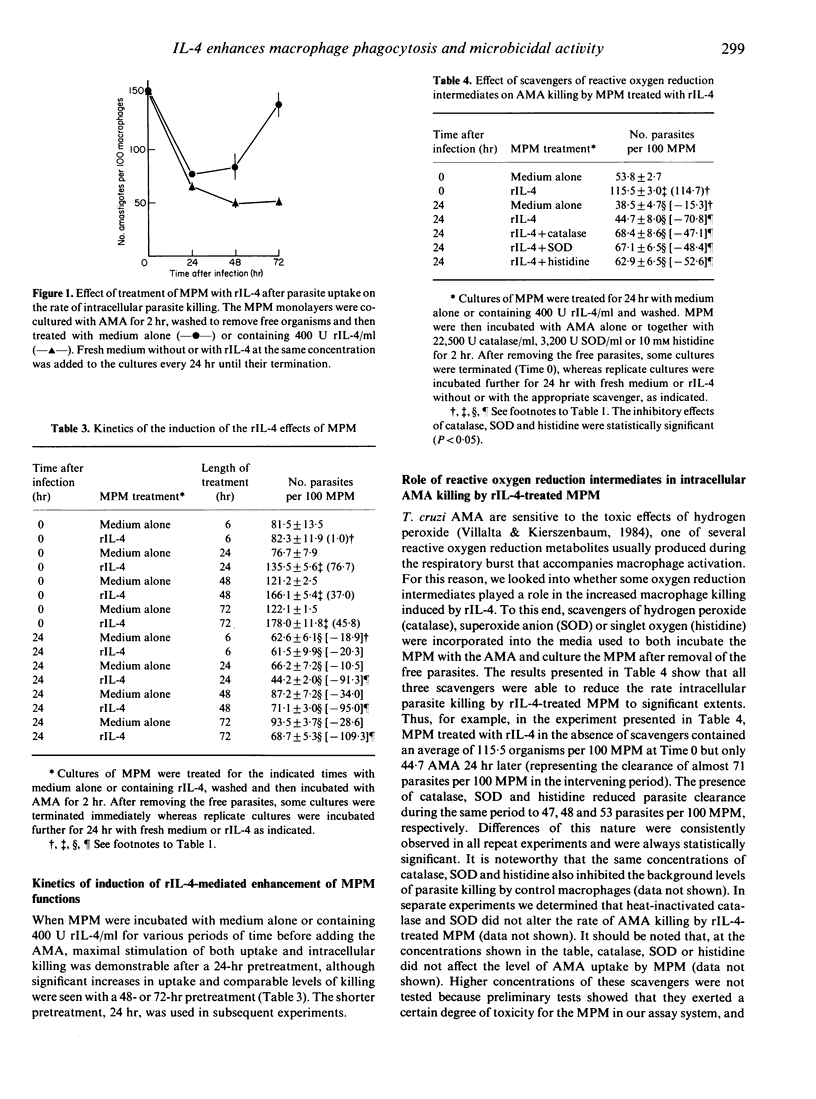
We studied whether interleukin-4 (IL-4) could modulate two macrophage functions relevant to their microbicidal activity (uptake and killing), using non-invasive [amastigote (AMA)] forms of the protozoan parasite Trypanosoma cruzi. Treatment of cultures of mouse resident peritoneal macrophages (MPM) with the supernatant of cultures of cells transfected with IL-4 cDNA increased both the capacity of the MPM to take up the organisms and the rate of intracellular killing with respect to MPM mock-treated with medium alone. The presence in the medium of a monoclonal antibody specific for IL-4 during MPM treatment inhibited both effects, pointing to recombinant IL-4 (rIL-4) as the active principle in the supernatant. Kinetic studies revealed that at least a 24-hr pretreatment of the MPM with the rIL-4-containing supernatant was required for these effects to be produced. The rate of intracellular parasite killing was also significantly increased when the rIL-4 treatment was applied after AMA ingestion by MPM. This result confirmed that MPM could be activated by rIL-4 for greater intracellular killing and showed that this enhancement was not necessarily dependent on the initial rIL-4-mediated increase in parasite load. The use of scavengers of reactive oxygen reduction intermediates indicated that hydrogen peroxide, superoxide anion and singlet oxygen, but apparently not hydroxyl radicals, were involved in parasite killing modulated by rIL-4. These results document for the first time the capacity of IL-4 to enhance the microbicidal activity of macrophages and suggest that this lymphokine might play a role in host defence against T. cruzi infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Zenian A., Wirth J. J. Macrophage activation by cord factor (trehalose 6,6'-dimycolate): enhanced association with and intracellular killing of Trypanosoma cruzi. Infect Immun. 1984 Feb;43(2):531–535. doi: 10.1128/iai.43.2.531-535.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987 Feb 5;325(6104):537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Goldberger G., Dinarello C. A., Mizel S. B., Colten H. R. Regulation of class III major histocompatibility complex gene products by interleukin-1. Science. 1986 May 16;232(4752):850–852. doi: 10.1126/science.3010455. [DOI] [PubMed] [Google Scholar]
- Plata F., Wietzerbin J., Pons F. G., Falcoff E., Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984 Oct;14(10):930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Jaffe E. A., Murray H. W. Oxygen-independent inhibition of intracellular Chlamydia psittaci growth by human monocytes and interferon-gamma-activated macrophages. J Immunol. 1986 Jul 15;137(2):689–692. [PubMed] [Google Scholar]
- Steeg P. S., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by an immune interferon-like lymphokine: inhibitory effect of endotoxin. J Immunol. 1982 Dec;129(6):2402–2406. [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Growth of isolated amastigotes of Trypanosoma cruzi in cell-free medium. J Protozool. 1982 Nov;29(4):570–576. doi: 10.1111/j.1550-7408.1982.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of inflammatory cells in Chagas' disease. II. Interactions of mouse macrophages and human monocytes with intracellular forms of Trypanosoma cruzi: uptake and mechanism of destruction. J Immunol. 1984 Dec;133(6):3338–3343. [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F., Sonnenfeld G., Zlotnik A. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect Immun. 1985 Jul;49(1):61–66. doi: 10.1128/iai.49.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Stimulatory effects of leukotriene B4 on macrophage association with and intracellular destruction of Trypanosoma cruzi. J Immunol. 1985 Mar;134(3):1989–1993. [PubMed] [Google Scholar]
- Zlotnik A., Fischer M., Roehm N., Zipori D. Evidence for effects of interleukin 4 (B cell stimulatory factor 1) on macrophages: enhancement of antigen presenting ability of bone marrow-derived macrophages. J Immunol. 1987 Jun 15;138(12):4275–4279. [PubMed] [Google Scholar]


