Abstract
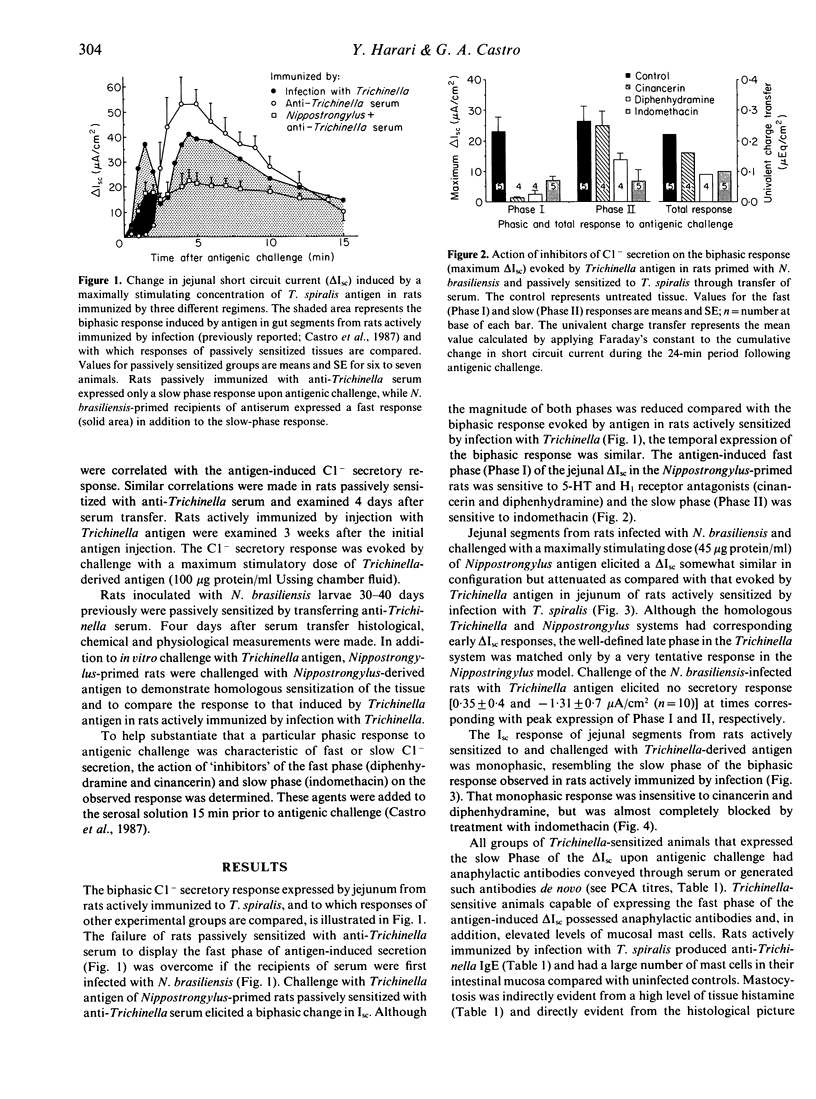
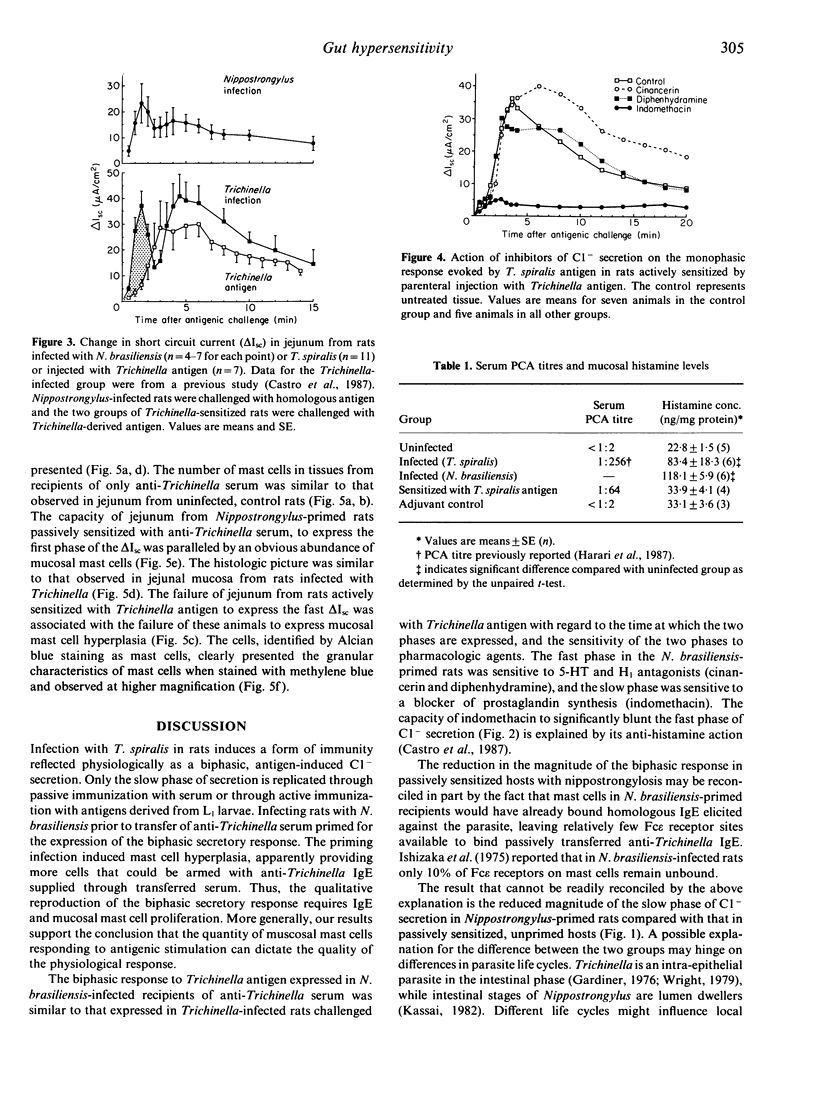
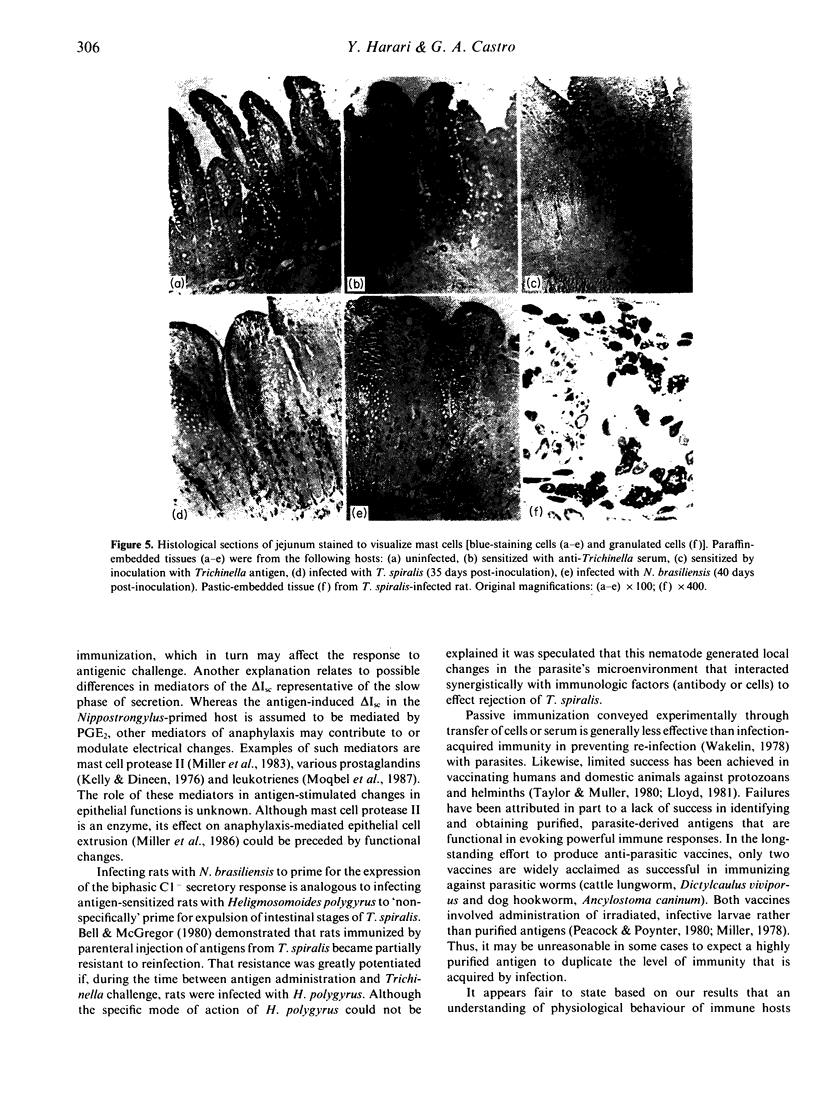
Antigenic challenge of jejunum from rats infected with Trichinella spiralis evokes a biphasic pattern of epithelial Cl- secretion, as measured in vitro by electrophysiological methods. Peaks of secretion occur at approximately 1.5 and approximately 5.0 min post-challenge. Challenge of jejunum from hosts passively immunized with serum containing anti-Trichinella anaphylactic antibody evokes the late phase but not the early phase of Cl- secretion. Since the early phase is mediated by 5-hydroxytryptamine and histamine from mast cells, we hypothesized that the failure to express that phase was due to a decrease in mast cell-derived mediators secondary to a deficiency in mucosal mast cell numbers. The hypothesis was tested by correlating mast cell numbers with patterns of antigen-induced Cl- secretion using several immunization regimes. Rats actively immunized by infection produced anti-Trichinella IgE and had a mucosal mastocytosis. Rats passively sensitized with serum containing anti-Trichinella IgE had normal numbers of mast cells in their mucosa. Inducing mastocytosis in rats, by infecting them with Nippostrongylus brasiliensis prior to passive sensitization with anti-Trichinella serum, primed for the expression of a biphasic Cl- secretory response upon subsequent challenge with Trichinella antigen. Rats actively sensitized by injection with Trichinella antigen elicited an IgE response without mastocytosis and expressed only the late phase of antigen-induced Cl- secretion. Results (i) support our hypothesis, (ii) emphasize the importance of the cellular state of the mucosa in the functional expression of local anaphylaxis; and (iii) provide a physiological explanation for the general failure of vaccination and passive sensitization to induce functional immunity equivalent to that induced by natural infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizadeh H., Wakelin D. Comparison of rapid expulsion of Trichinella spiralis in mice and rats. Int J Parasitol. 1982 Feb;12(1):65–73. doi: 10.1016/0020-7519(82)90097-2. [DOI] [PubMed] [Google Scholar]
- Baird A. W., Coombs R. R., McLaughlan P., Cuthbert A. W. Immediate hypersensitivity reactions to cow milk proteins in isolated epithelium from ileum of milk-drinking guinea-pigs: comparisons with colonic epithelia. Int Arch Allergy Appl Immunol. 1984;75(3):255–263. doi: 10.1159/000233625. [DOI] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D. Rapid expulsion of Trichinella spiralis: coinduction by using antigenic extracts of larvae and intestinal stimulation with an unrelated parasite. Infect Immun. 1980 Jul;29(1):194–199. doi: 10.1128/iai.29.1.194-199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro G. A., Fairbairn D. Carbohydrates and lipids in Trichinella spiralis larvae and their utilization in vitro. J Parasitol. 1969 Feb;55(1):51–58. [PubMed] [Google Scholar]
- Castro G. A., Fairbairn D. Effect of immune serum on glucose absorption and infectivity of Trichinella spiralis. J Parasitol. 1969 Feb;55(1):59–66. [PubMed] [Google Scholar]
- Castro G. A., Harari Y., Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987 Oct;253(4 Pt 1):G540–G548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- Gardiner C. H. Habitat and reproductive behavior of Trichinella spiralis. J Parasitol. 1976 Dec;62(6):865–870. [PubMed] [Google Scholar]
- Harari Y., Russell D. A., Castro G. A. Anaphylaxis-mediated epithelial Cl- secretion and parasite rejection in rat intestine. J Immunol. 1987 Feb 15;138(4):1250–1255. [PubMed] [Google Scholar]
- Ishizaka T., König W., Kurata M., Mauser L., Ishizaka K. Immunologic properties of mast cells from rats infected with Nippostrongylus brasiliensis. J Immunol. 1975 Oct;115(4):1078–1083. [PubMed] [Google Scholar]
- Kelly J. D., Dineen J. K. Prostaglandins in the gastrointestinal tract: evidence for a role in worm expulsion. Aust Vet J. 1976 Sep;52(9):391–397. doi: 10.1111/j.1751-0813.1976.tb09510.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd S. Progress in immunization against parasitic helminths. Parasitology. 1981 Aug;83(Pt 1):225–242. doi: 10.1017/s0031182000050186. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Woodbury R. G., Huntley J. F., Newlands G. Systemic release of mucosal mast-cell protease in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983 Jul;49(3):471–479. [PMC free article] [PubMed] [Google Scholar]
- Miller T. A. Industrial development and field use of the canine hookworm vaccine. Adv Parasitol. 1978;16:333–342. doi: 10.1016/s0065-308x(08)60577-1. [DOI] [PubMed] [Google Scholar]
- Moqbel R., Wakelin D., MacDonald A. J., King S. J., Grencis R. K., Kay A. B. Release of leukotrienes during rapid expulsion of Trichinella spiralis from immune rats. Immunology. 1987 Mar;60(3):425–430. [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WELLS P. D. Mast cell, eosinophil and histamine levels in Nippostrongylus brasiliensis infected rats. Exp Parasitol. 1962 Apr;12:82–101. doi: 10.1016/s0014-4894(62)80002-2. [DOI] [PubMed] [Google Scholar]
- Wakelin D. Immunity to intestinal parasites. Nature. 1978 Jun 22;273(5664):617–620. doi: 10.1038/273617a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Ovary Z. Antigen and antibody detection by in vivo methods; a reevaluation of passive cutaneous anaphylactic reactions. J Immunol Methods. 1977;14(3-4):381–390. doi: 10.1016/0022-1759(77)90149-1. [DOI] [PubMed] [Google Scholar]
- Wingren U., Enerbäck L., Ahlman H., Allenmark S., Dahlström A. Amines of the mucosal mast cell of the gut in normal and nematode infected rats. Histochemistry. 1983;77(2):145–158. doi: 10.1007/BF00506557. [DOI] [PubMed] [Google Scholar]
- Wright K. A. Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol. 1979 Jun;65(3):441–445. [PubMed] [Google Scholar]



