Abstract
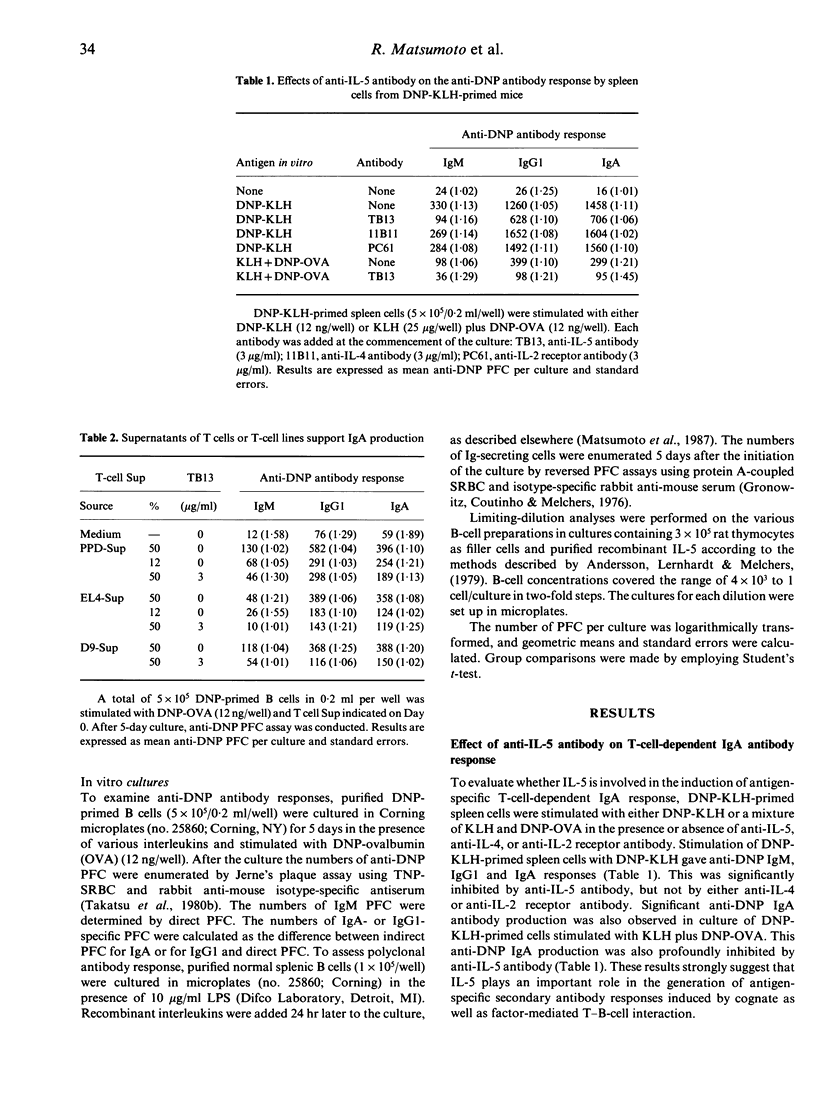
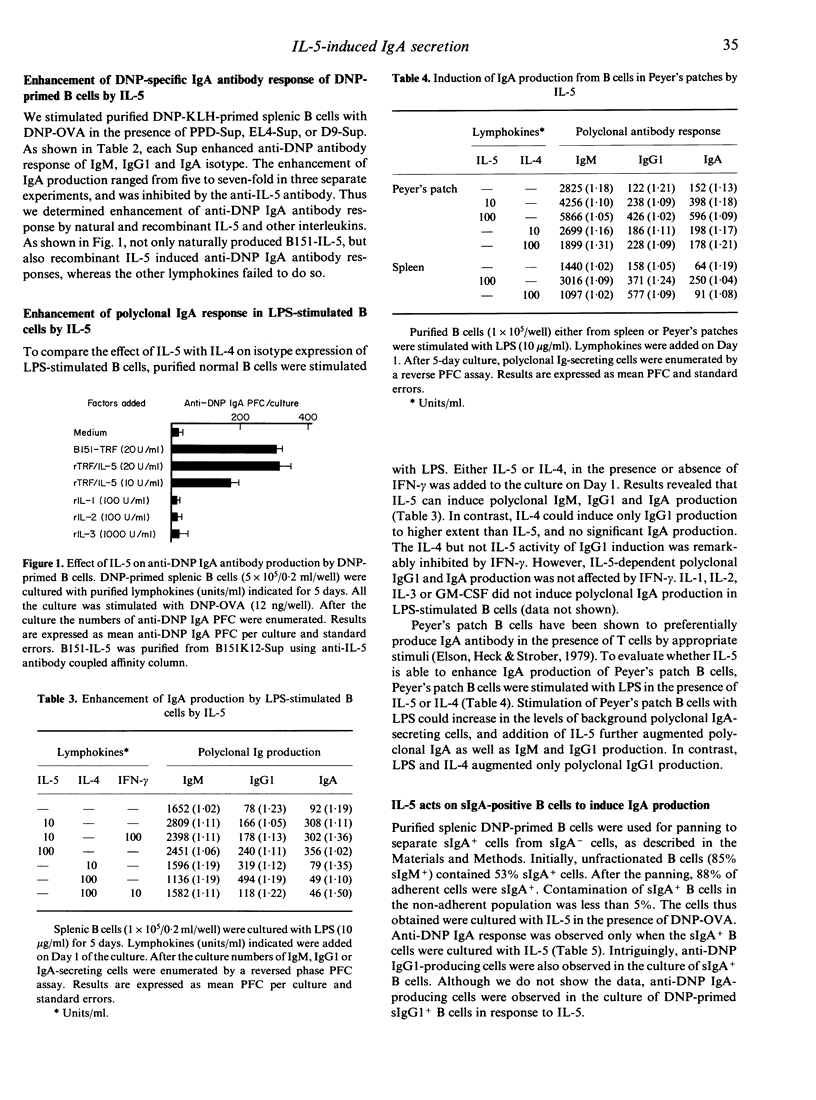
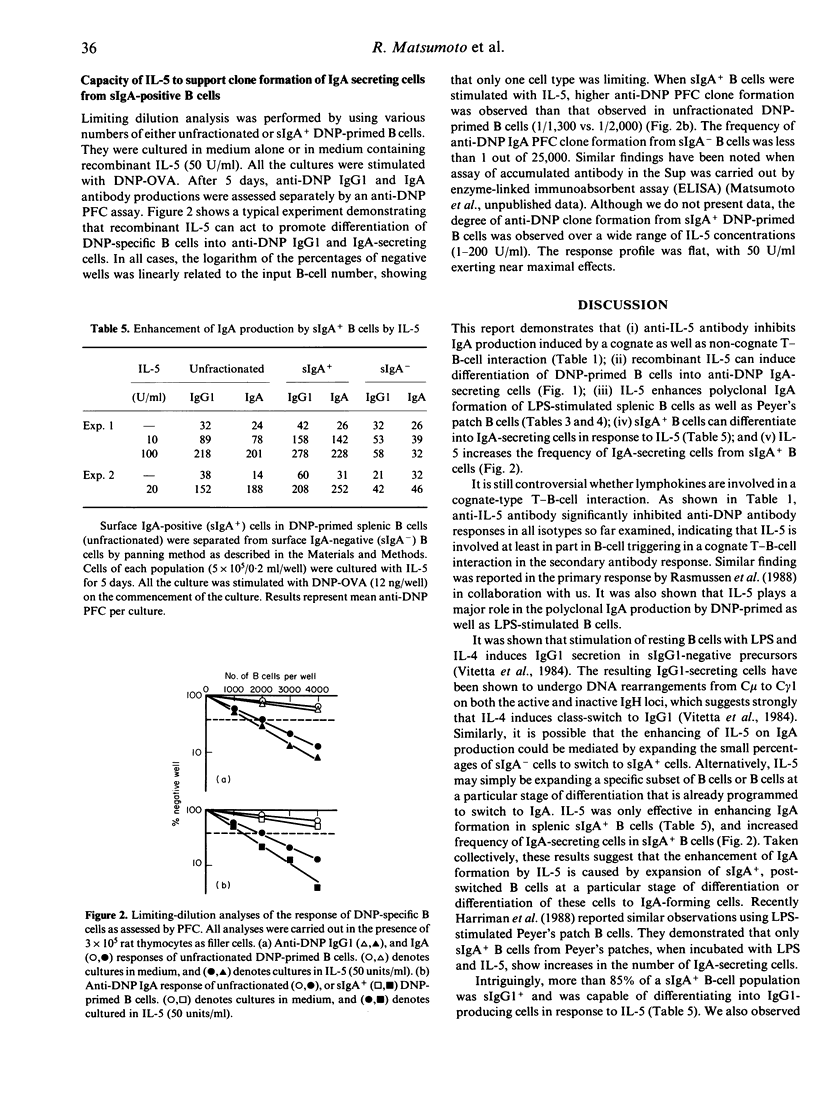
There is a body of evidence suggesting that IgA production is regulated by helper T cells or their products. To elucidate molecular mechanisms of IgA production, the role of lymphokines in the in vitro antigen-specific and polyclonal IgA responses was examined. Supernatants from antigen-stimulated T cells or mitogen-stimulated T-cell clones can enhance 2,4-dinitro phenyl (DNP)-specific IgM, IgG1 and IgA production in cultures of DNP-ovalbumin (OVA)-stimulated T-cell-depleted spleen cells from DNP-keyhole limpet haemocyanin-primed mice. The IgA enhancement was inhibited by anti-IL-5 monoclonal antibody. Purified recombinant IL-5 could also enhance anti-DNP IgA production in a dose-dependent manner. This enhancing effect was not substituted by IL-1, IL-2, IL-3 or IL-4. Polyclonal IgA secretion of lipopolysaccharide-stimulated normal B cells was augmented preferentially by IL-5, but not by IL-4. Surface IgA-positive (sIgA+) B cells, but not surface IgA-negative B cells, responded to IL-5 for the development of IgA-secreting cells. Limiting-dilution analysis revealed that IL-5 increases the frequency of IgA-secreting cells in sIgA+ B-cell populations. These results indicate that IL-5 plays an essential role in the antigen-specific and polyclonal IgA formation as a maturation-inducing factor rather than class-switching factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. The switch from IgM to IgG secretion in single mitogen-stimulated B-cell clones. J Exp Med. 1978 Jun 1;147(6):1744–1754. doi: 10.1084/jem.147.6.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Lernhardt W., Melchers F. The purified protein derivative of turberculin, a B-cell mitogen that distinguishes in its action resting, small B cells from activated B-cell blasts. J Exp Med. 1979 Dec 1;150(6):1339–1350. doi: 10.1084/jem.150.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstedt-Lindqvist S., Sideras P., MacDonald H. R., Severinson E. Regulation of Ig class secretion by soluble products of certain T-cell lines. Immunol Rev. 1984 Apr;78:25–50. doi: 10.1111/j.1600-065x.1984.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Bond M. W., Shrader B., Mosmann T. R., Coffman R. L. A mouse T cell product that preferentially enhances IgA production. II. Physicochemical characterization. J Immunol. 1987 Dec 1;139(11):3691–3696. [PubMed] [Google Scholar]
- Cebra J. J., Komisar J. L., Schweitzer P. A. CH isotype 'switching' during normal B-lymphocyte development. Annu Rev Immunol. 1984;2:493–548. doi: 10.1146/annurev.iy.02.040184.002425. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Coffman R. L., Shrader B., Carty J., Mosmann T. R., Bond M. W. A mouse T cell product that preferentially enhances IgA production. I. Biologic characterization. J Immunol. 1987 Dec 1;139(11):3685–3690. [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Kincade P. W. A two-stage model for development of antibody-producing cells. Clin Exp Immunol. 1972 May;11(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Heck J. A., Strober W. T-cell regulation of murine IgA synthesis. J Exp Med. 1979 Mar 1;149(3):632–643. doi: 10.1084/jem.149.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Harada N., Kikuchi Y., Tominaga A., Takaki S., Takatsu K. BCGFII activity on activated B cells of a purified murine T cell-replacing factor (TRF) from a T cell hybridoma (B151K12). J Immunol. 1985 Jun;134(6):3944–3951. [PubMed] [Google Scholar]
- Harada N., Takahashi T., Matsumoto M., Kinashi T., Ohara J., Kikuchi Y., Koyama N., Severinson E., Yaoita Y., Honjo T. Production of a monoclonal antibody useful in the molecular characterization of murine T-cell-replacing factor/B-cell growth factor II. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4581–4585. doi: 10.1073/pnas.84.13.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriman G. R., Kunimoto D. Y., Elliott J. F., Paetkau V., Strober W. The role of IL-5 in IgA B cell differentiation. J Immunol. 1988 May 1;140(9):3033–3039. [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983 Feb 1;157(2):433–450. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. II. Terminal differentiation of postswitch sIgA-bearing Peyer's patch B cells. J Exp Med. 1983 Sep 1;158(3):649–669. doi: 10.1084/jem.158.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. Factors affecting B-cell growth and differentiation. Annu Rev Immunol. 1985;3:133–157. doi: 10.1146/annurev.iy.03.040185.001025. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Ishizaka K. Regulation of antibody response in vitro. IX. Induction of secondary anti-hapten IgG antibody response by anti-immunoglobulin and enhancing soluble factor. J Immunol. 1975 Feb;114(2 Pt 1):585–591. [PubMed] [Google Scholar]
- Kishimoto T., Ishizaka K. Regulation of antibody response in vitro. VII. Enhancing soluble factors for IgG and IgE antibody response. J Immunol. 1973 Oct;111(4):1194–1205. [PubMed] [Google Scholar]
- Koyama N., Harada N., Takahashi T., Mita S., Okamura H., Tominaga A., Takatsu K. Role of recombinant interleukin-1 compared to recombinant T-cell replacing factor/interleukin-5 in B-cell differentiation. Immunology. 1988 Feb;63(2):277–283. [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Tominaga A., Harada N., Takatsu K. Role of T cell-replacing factor (TRF) in the murine B cell differentiation: induction of increased levels of expression of secreted type IgM mRNA. J Immunol. 1987 Mar 15;138(6):1826–1833. [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Kunkel H. G. Human T cell hybridomas secreting factors for IgA-specific help, polyclonal B cell activation, and B cell proliferation. J Exp Med. 1982 Dec 1;156(6):1860–1865. doi: 10.1084/jem.156.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie D. T., Filutowicz H. I., Swain S. L., Dutton R. W. Purification and partial sequence analysis of murine B cell growth factor II (interleukin 5). J Immunol. 1987 Oct 15;139(8):2661–2668. [PubMed] [Google Scholar]
- McNabb P. C., Tomasi T. B. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol. 1981;35:477–496. doi: 10.1146/annurev.mi.35.100181.002401. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Murray P. D., McKenzie D. T., Swain S. L., Kagnoff M. F. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987 Oct 15;139(8):2669–2674. [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Okumura K., Julius M. H., Tsu T., Herzenberg L. A., Herzenberg L. A. Demonstration that IgG memory is carried by IgG-bearing cells. Eur J Immunol. 1976 Jul;6(7):467–472. doi: 10.1002/eji.1830060704. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Perlmutter A. P., Gilbert W. Antibodies of the secondary response can be expressed without switch recombination in normal mouse B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7189–7193. doi: 10.1073/pnas.81.22.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen R., Takatsu K., Harada N., Takahashi T., Bottomly K. T cell-dependent hapten-specific and polyclonal B cell responses require release of interleukin 5. J Immunol. 1988 Feb 1;140(3):705–712. [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Swain S. L. Role of BCGFII in the differentiation to antibody secretion normal and tumor B cells. J Immunol. 1985 Jun;134(6):3934–3943. [PubMed] [Google Scholar]
- Takatsu K., Harada N., Hara Y., Takahama Y., Yamada G., Dobashi K., Hamaoka T. Purification and physicochemical characterization of murine T cell replacing factor (TRF). J Immunol. 1985 Jan;134(1):382–389. [PubMed] [Google Scholar]
- Takatsu K., Tanaka K., Tominaga A., Kumahara Y., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). III. Establishment of T cell hybrid clone continuously producing TRF and functional analysis of released TRF. J Immunol. 1980 Dec;125(6):2646–2653. [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). I. Functional characterization of a TRF-producing helper T cell subset and genetic studies on TRF production. J Immunol. 1980 May;124(5):2414–2422. [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Harada N., Mita S., Matsumoto M., Takahashi T., Kikuchi Y., Yamaguchi N. T cell-replacing factor (TRF)/interleukin 5 (IL-5): molecular and functional properties. Immunol Rev. 1988 Feb;102:107–135. doi: 10.1111/j.1600-065x.1988.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Teale J. M., Lafrenz D., Klinman N. R., Strober S. Immunoglobulin class commitment exhibited by B lymphocytes separated according to surface isotype. J Immunol. 1981 May;126(5):1952–1957. [PubMed] [Google Scholar]
- Tominaga A., Matsumoto M., Harada N., Takahashi T., Kikuchi Y., Takatsu K. Molecular properties and regulation of mRNA expression for murine T cell-replacing factor/IL-5. J Immunol. 1988 Feb 15;140(4):1175–1181. [PubMed] [Google Scholar]
- Vitetta E. S., Brooks K., Chen Y. W., Isakson P., Jones S., Layton J., Mishra G. C., Pure E., Weiss E., Word C. T-cell-derived lymphokines that induce IgM and IgG secretion in activated murine B cells. Immunol Rev. 1984 Apr;78:137–157. doi: 10.1111/j.1600-065x.1984.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Kumagai Y., Okumura K., Honjo T. Expression of lymphocyte surface IgE does not require switch recombination. Nature. 1982 Jun 24;297(5868):697–699. doi: 10.1038/297697a0. [DOI] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan-Bar I., Strober S., Vitetta E. S. The relationship between surface immunoglobulin isotype and immune function of murine B lymphocytes. I. Surface immunoglobulin isotypes on primed B cells in the spleen. J Exp Med. 1977 May 1;145(5):1188–1205. doi: 10.1084/jem.145.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan-Bar I., Vitetta E. S., Assisi F., Strober S. The relationship between surface immunoglobulin isotype and immune function of murine B lymphocytes. III. Expression of a single predominant isotype on primed and unprimed B cells. J Exp Med. 1978 May 1;147(5):1374–1394. doi: 10.1084/jem.147.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]


