Abstract
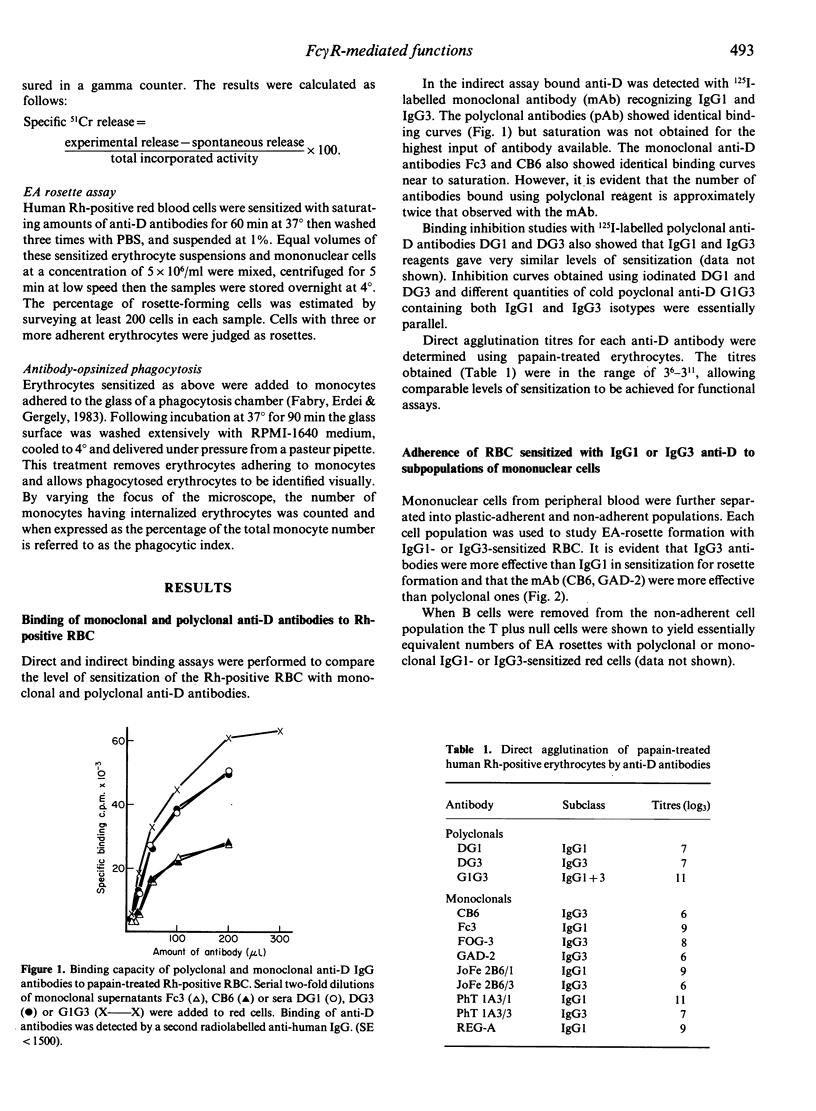
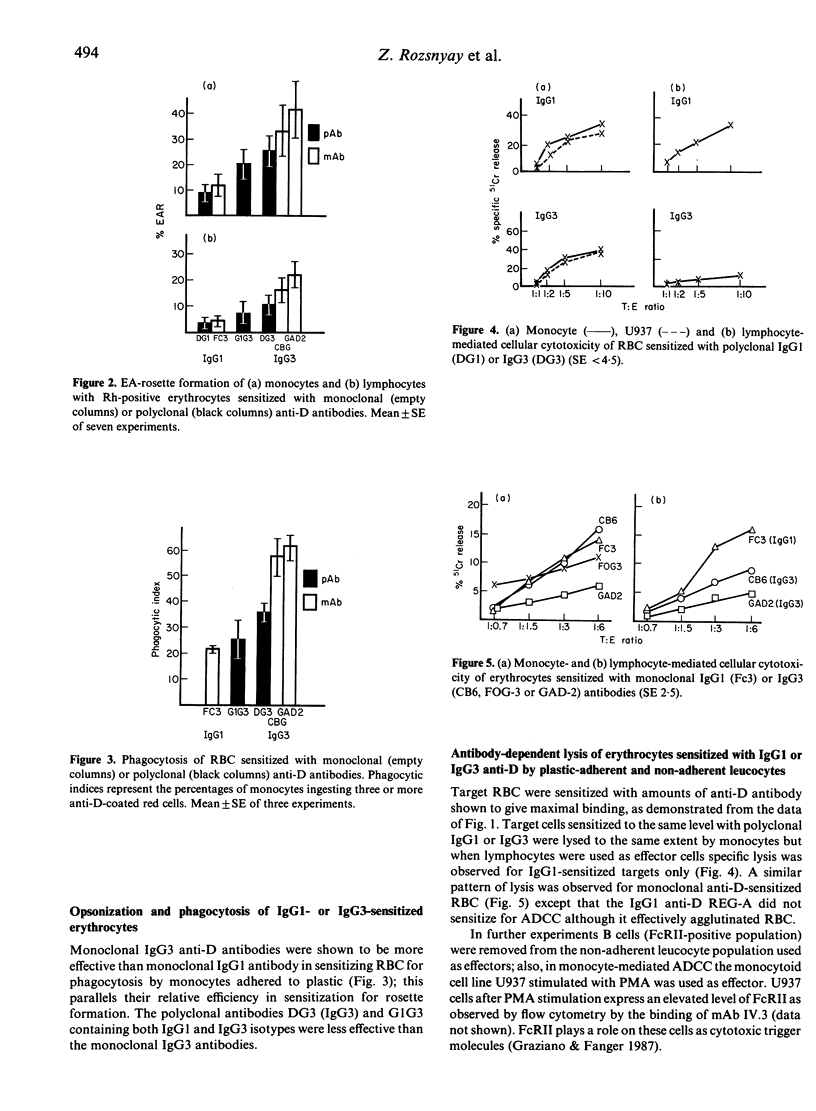
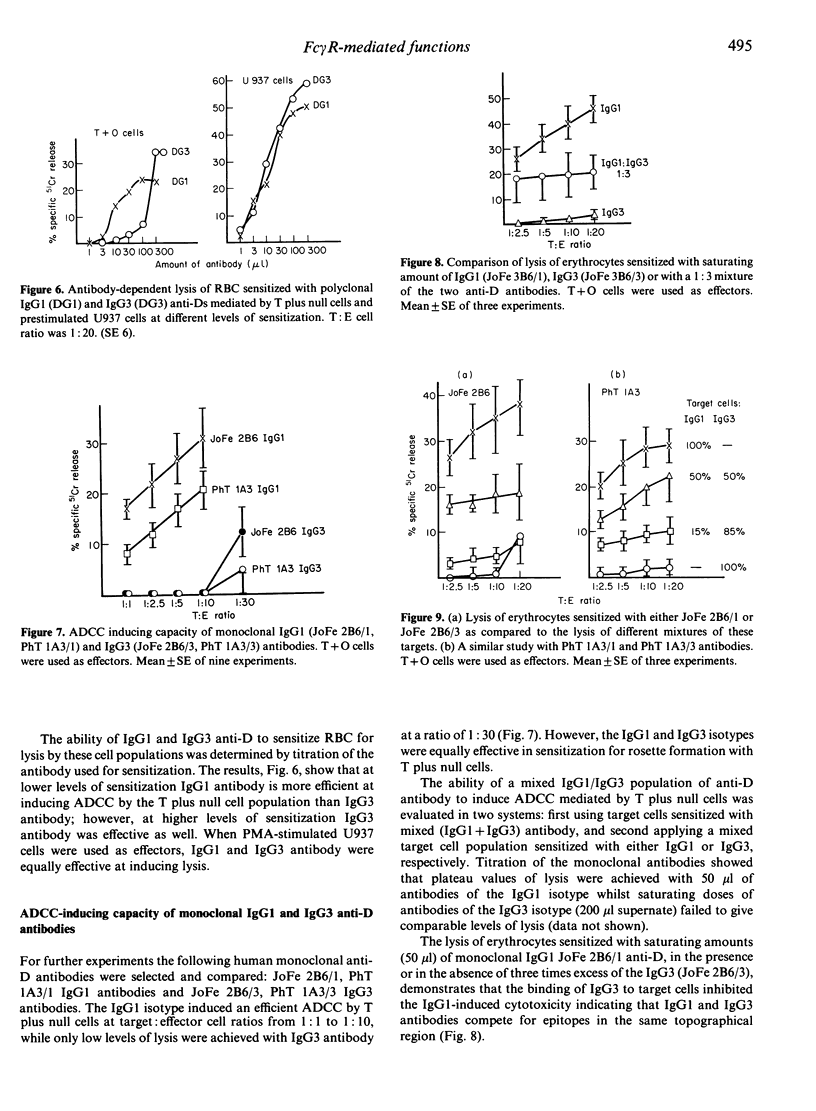
Polyclonal and monoclonal anti-Rh (D) antibodies of IgG1 and IgG3 subclass were evaluated for their capacity to sensitize erythrocytes and (i) to trigger monocyte and K-cell mediated antibody-dependent cellular cytotoxicity (ADCC); (ii) to mediate binding to monocyte and lymphocyte Fc gamma R; (iii) to stimulate phagocytosis by monocytes. All antibodies were equally effective in mediating monocyte or activated U937 cell ADCC but IgG1 was more active than IgG3 in K-cell mediated ADCC. IgG3-sensitized erythrocytes inhibited IgG1-induced lysis, suggesting that each subclass engages the same Fc gamma R receptor but that lysis requires a further 'signal' that the IgG3 molecule can not deliver. Two monoclonal IgG3 anti-D antibodies were shown to have higher binding (two times) and phagocytic (three times) indices than IgG1 antibody for monocytes; similar differences were observed for polyclonal IgG1 and IgG3 antibodies. The same pattern was observed in an EA rosette assay when a total lymphocyte population was used; however, this difference was not seen with a B-cell depleted (T+ null cell) lymphocyte population.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aman P., Ehlin-Henriksson B., Klein G. Epstein-Barr virus susceptibility of normal human B lymphocyte populations. J Exp Med. 1984 Jan 1;159(1):208–220. doi: 10.1084/jem.159.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay G. R., Forouhi P., McCann M. C., Greiss M. A., Urbaniak S. J. ADCC lysis of human erythrocytes sensitized with rhesus alloantibodies. IV. Characterization of anti-D sera which are inactive in ADCC. Br J Haematol. 1985 Jun;60(2):293–304. doi: 10.1111/j.1365-2141.1985.tb07415.x. [DOI] [PubMed] [Google Scholar]
- Benczur M., Györffy G., Garam T., Varga M., Medgyesi G., Sándor M., Petranyi G. G. Correlation between effector lymphocytes in natural and antibody-mediated cytotoxicity. Immunobiology. 1979 Sep;156(3):320–329. [PubMed] [Google Scholar]
- Brüggemann M., Williams G. T., Bindon C. I., Clark M. R., Walker M. R., Jefferis R., Waldmann H., Neuberger M. S. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987 Nov 1;166(5):1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B. Lymphocyte receptors for immunoglobulin. Adv Immunol. 1976;24:167–214. doi: 10.1016/s0065-2776(08)60330-2. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., Selvendran Y., Murdoch S., Palmer D. G., Hogg N. The human mononuclear phagocyte high-affinity Fc receptor, FcRI, defined by a monoclonal antibody, 10.1. Eur J Immunol. 1987 Oct;17(10):1453–1459. doi: 10.1002/eji.1830171011. [DOI] [PubMed] [Google Scholar]
- Doyle A., Jones T. J., Bidwell J. L., Bradley B. A. In vitro development of human monoclonal antibody-secreting plasmacytomas. Hum Immunol. 1985 Jul;13(3):199–209. doi: 10.1016/0198-8859(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Fábry Z., Erdei A., Gergely J. C3b acceptors on macrophages: inhibition of Fc gamma-receptor-mediated phagocytosis by acceptor-bound C3b. Immunol Lett. 1983 Jun;6(6):287–291. doi: 10.1016/0165-2478(83)90068-8. [DOI] [PubMed] [Google Scholar]
- Graziano R. F., Fanger M. W. Fc gamma RI and Fc gamma RII on monocytes and granulocytes are cytotoxic trigger molecules for tumor cells. J Immunol. 1987 Nov 15;139(10):3536–3541. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Looney R. J., Anderson C. L. Two distinct classes of IgG Fc receptors on a human monocyte line (U937) defined by differences in binding of murine IgG subclasses at low ionic strength. J Immunol. 1985 Nov;135(5):3348–3353. [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinaud J., Blanc M., Grandjean H., Fournie A., Bierme S., Pontonnier G. IgG subclasses and Gm allotypes of anti-D antibodies during pregnancy: correlation with the gravity of the fetal disease. Am J Obstet Gynecol. 1985 Apr 15;151(8):1111–1115. doi: 10.1016/0002-9378(85)90393-x. [DOI] [PubMed] [Google Scholar]
- Partridge L. J., Woof J. M., Jefferis R., Burton D. R. The use of anti-IgG monoclonal antibodies in mapping the monocyte receptor site on IgG. Mol Immunol. 1986 Dec;23(12):1365–1372. doi: 10.1016/0161-5890(86)90022-2. [DOI] [PubMed] [Google Scholar]
- Sármay G., Benczur M., Petrányi G., Klein E., Kahn M., Stanworth D. R., Gergely J. Ligand inhibition studies on the role of Fc receptors in antibody-dependent cell-mediated cytotoxicity. Mol Immunol. 1984 Jan;21(1):43–51. doi: 10.1016/0161-5890(84)90088-9. [DOI] [PubMed] [Google Scholar]
- Sármay G., Jefferis R., Gergely J. CH2 and CH3 domain deleted IgG1 paraproteins inhibit differently Fc receptor mediated binding and cytolysis. Immunol Lett. 1986 Jun;12(5-6):307–312. doi: 10.1016/0165-2478(86)90035-0. [DOI] [PubMed] [Google Scholar]
- Sármay G., Jefferis R., Klein E., Benczur M., Gergely J. Mapping the functional topography of Fc gamma with monoclonal antibodies: localization of epitopes interacting with the binding sites of Fc receptor on human K cells. Eur J Immunol. 1985 Oct;15(10):1037–1042. doi: 10.1002/eji.1830151015. [DOI] [PubMed] [Google Scholar]
- Thompson K. M., Hough D. W., Maddison P. J., Melamed M. D., Hughes-Jones N. The efficient production of stable, human monoclonal antibody-secreting hybridomas from EBV-transformed lymphocytes using the mouse myeloma X63-Ag8.653 as a fusion partner. J Immunol Methods. 1986 Nov 20;94(1-2):7–12. doi: 10.1016/0022-1759(86)90208-5. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., O'Brien T., Shade M., Perussia B. Phorbol esters enhance spontaneous cytotoxicity of human lymphocytes, abrogate Fc receptor expression, and inhibit antibody-dependent lymphocyte-mediated cytotoxicity. J Immunol. 1984 Oct;133(4):1869–1877. [PubMed] [Google Scholar]
- Urbaniak S. J., Greiss M. A. ADCC (K-cell) lysis of human erythrocytes sensitized with rhesus alloantibodies. III. Comparison of IgG anti-D agglutinating and lytic (ADCC) activity and the role of IgG subclasses. Br J Haematol. 1980 Nov;46(3):447–453. doi: 10.1111/j.1365-2141.1980.tb05992.x. [DOI] [PubMed] [Google Scholar]
- Walker M. R., Kumpel B. M., Thompson K., Woof J. M., Burton D. R., Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins binding of human monoclonal anti-D antibodies to FcRI on the monocyte-like U937 cell line. Vox Sang. 1988;55(4):222–228. doi: 10.1111/j.1423-0410.1988.tb04701.x. [DOI] [PubMed] [Google Scholar]
- Wiener E., Atwal A., Thompson K. M., Melamed M. D., Gorick B., Hughes-Jones N. C. Differences between the activities of human monoclonal IgG1 and IgG3 subclasses of anti-D(Rh) antibody in their ability to mediate red cell-binding to macrophages. Immunology. 1987 Nov;62(3):401–404. [PMC free article] [PubMed] [Google Scholar]
- Woof J. M., Nik Jaafar M. I., Jefferis R., Burton D. R. The monocyte binding domain(s) on human immunoglobulin G. Mol Immunol. 1984 Jun;21(6):523–527. doi: 10.1016/0161-5890(84)90068-3. [DOI] [PubMed] [Google Scholar]
- Woof J. M., Partridge L. J., Jefferis R., Burton D. R. Localisation of the monocyte-binding region on human immunoglobulin G. Mol Immunol. 1986 Mar;23(3):319–330. doi: 10.1016/0161-5890(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Yust I., Frisch B., Goldsher N. Antibody-dependent, cell-mediated cytotoxicity against human red blood cells: correlation of effector cell type with enzymatic alteration of the target cell surface. Eur J Immunol. 1980 Feb;10(2):127–131. doi: 10.1002/eji.1830100211. [DOI] [PubMed] [Google Scholar]
- Zupańska B., Thomson E. E., Merry A. H. Fc receptors for IgG1 and IgG3 on human mononuclear cells--an evaluation with known levels of erythrocyte-bound IgG. Vox Sang. 1986;50(2):97–103. doi: 10.1111/j.1423-0410.1986.tb04854.x. [DOI] [PubMed] [Google Scholar]



