Abstract
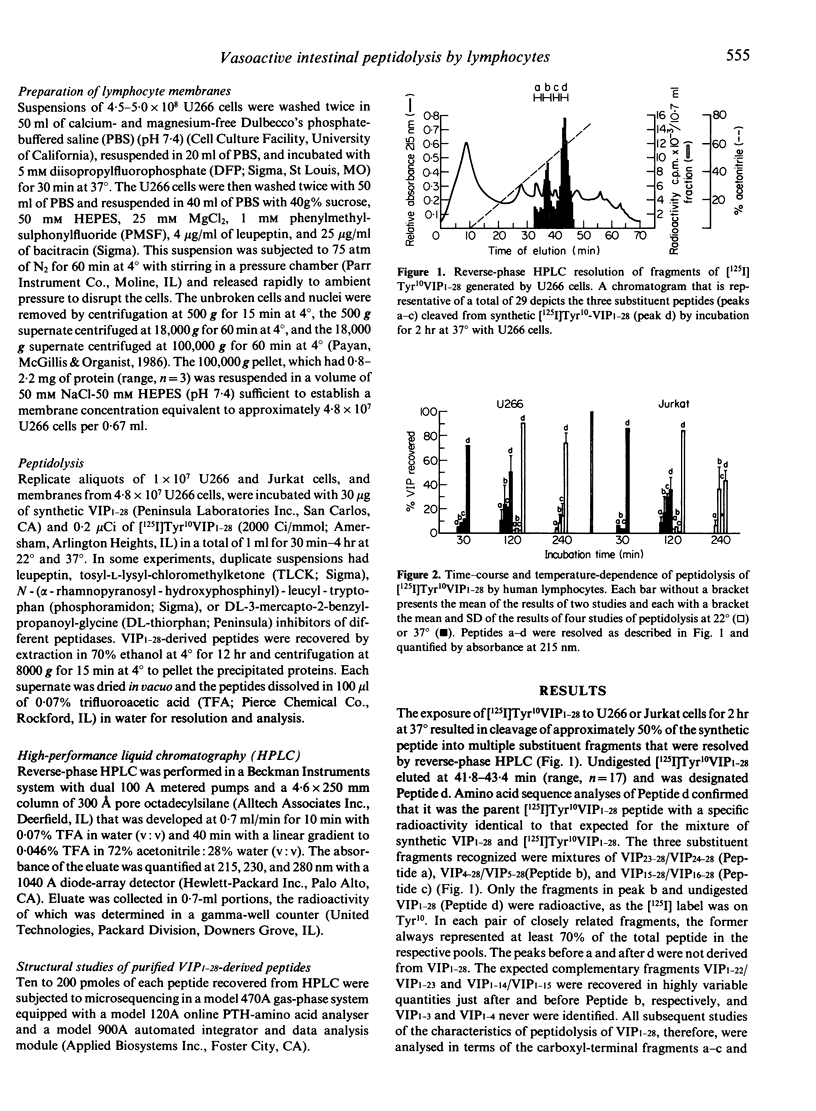
Human cultured T lymphocytes of the Jurkat line and myeloma cells of the U266 line cleaved the 28 amino acid vasoactive intestinal peptide (VIP1-28) preferentially at three sites with time- and temperature-dependence. The fragments VIP4-28 and VIP23-28) from an endopeptidase activity, and VIP15-28 from a trypsin-like peptidase, together represented a range of 26-65% of the VIP1-28 recovered after 2 hr at 37 degrees C or 4 hr at 22 degrees C, based on the absorbance of purified peptides and the radioactivity of [125I]Tyr10 VIP1-28. The endopeptidase activity was associated with membranes recovered after disruption of U266 cells by nitrogen cavitation. Pretreatment of intact U266 and Jurkat cells with diisopropylfluorophosphate (DFP) and the subsequently isolated subcellular particles with phenylmethylsulphonylfluoride (PMSF) and leupeptin inhibited the trypsin-like enzyme by a mean of 80%, without suppressing endopeptidase activity. In contrast, 0.1 mM DL-thiorphan and phosphoramidon blocked selectively a range of 35-70% of the endopeptidase activity in membrane preparations and intact cells. The capacity of lymphocytes to degrade VIP1-28 may substantially alter the effects of this neuromediator on functions of some subsets of T and B cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliakbari J., Sreedharan S. P., Turck C. W., Goetzl E. J. Selective localization of vasoactive intestinal peptide and substance P in human eosinophils. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1440–1445. doi: 10.1016/s0006-291x(87)80293-0. [DOI] [PubMed] [Google Scholar]
- Altstein M., Bachar E., Vogel Z., Blumberg S. Protection of enkephalins from enzymatic degradation utilizing selective metal-chelating inhibitors. Eur J Pharmacol. 1983 Aug 5;91(4):353–361. doi: 10.1016/0014-2999(83)90158-9. [DOI] [PubMed] [Google Scholar]
- Calvo J. R., Molinero P., Jimenez J., Goberna R., Guerrero J. M. Interaction of vasoactive intestinal peptide (VIP) with rat lymphoid cells. Peptides. 1986 Mar-Apr;7(2):177–181. doi: 10.1016/0196-9781(86)90209-3. [DOI] [PubMed] [Google Scholar]
- Caughey G. H., Leidig F., Viro N. F., Nadel J. A. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther. 1988 Jan;244(1):133–137. [PubMed] [Google Scholar]
- Danek A., O'Dorisio M. S., O'Dorisio T. M., George J. M. Specific binding sites for vasoactive intestinal polypeptide on nonadherent peripheral blood lymphocytes. J Immunol. 1983 Sep;131(3):1173–1177. [PubMed] [Google Scholar]
- Goetzl E. J., Sreedharan S. P., Turck C. W. Structurally distinctive vasoactive intestinal peptides from rat basophilic leukemia cells. J Biol Chem. 1988 Jul 5;263(19):9083–9086. [PubMed] [Google Scholar]
- Jenne D., Rey C., Haefliger J. A., Qiao B. Y., Groscurth P., Tschopp J. Identification and sequencing of cDNA clones encoding the granule-associated serine proteases granzymes D, E, and F of cytolytic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4814–4818. doi: 10.1073/pnas.85.13.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Fahrenkrug J., Hökfelt T., Martling C. R., Larsson O., Tatemoto K., Anggård A. Co-existence of peptide HI (PHI) and VIP in nerves regulating blood flow and bronchial smooth muscle tone in various mammals including man. Peptides. 1984 May-Jun;5(3):593–606. doi: 10.1016/0196-9781(84)90090-1. [DOI] [PubMed] [Google Scholar]
- O'Dorisio M. S., O'Dorisio T. M., Cataland S., Balcerzak S. P. Vasoactive intestinal polypeptide as a biochemical marker for polymorphonuclear leukocytes. J Lab Clin Med. 1980 Oct;96(4):666–672. [PubMed] [Google Scholar]
- O'Dorisio M. S., Wood C. L., O'Dorisio T. M. Vasoactive intestinal peptide and neuropeptide modulation of the immune response. J Immunol. 1985 Aug;135(2 Suppl):792s–796s. [PubMed] [Google Scholar]
- O'Dorisio M. S., Wood C. L., Wenger G. D., Vassalo L. M. Cyclic AMP-dependent protein kinase in Molt 4b lymphoblasts: identification by photoaffinity labeling and activation in intact cells by vasoactive intestinal polypeptide (VIP) and peptide histidine isoleucine (PHI). J Immunol. 1985 Jun;134(6):4078–4086. [PubMed] [Google Scholar]
- Ottaway C. A., Greenberg G. R. Interaction of vasoactive intestinal peptide with mouse lymphocytes: specific binding and the modulation of mitogen responses. J Immunol. 1984 Jan;132(1):417–423. [PubMed] [Google Scholar]
- Ottaway C. A. Selective effects of vasoactive intestinal peptide on the mitogenic response of murine T cells. Immunology. 1987 Oct;62(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Pasternack M. S., Eisen H. N. A novel serine esterase expressed by cytotoxic T lymphocytes. 1985 Apr 25-May 1Nature. 314(6013):743–745. doi: 10.1038/314743a0. [DOI] [PubMed] [Google Scholar]
- Payan D. G., McGillis J. P., Organist M. L. Binding characteristics and affinity labeling of protein constituents of the human IM-9 lymphoblast receptor for substance P. J Biol Chem. 1986 Oct 25;261(30):14321–14329. [PubMed] [Google Scholar]
- Wood C. L., O'Dorisio M. S. Covalent cross-linking of vasoactive intestinal polypeptide to its receptors on intact human lymphoblasts. J Biol Chem. 1985 Jan 25;260(2):1243–1247. [PubMed] [Google Scholar]


