Abstract
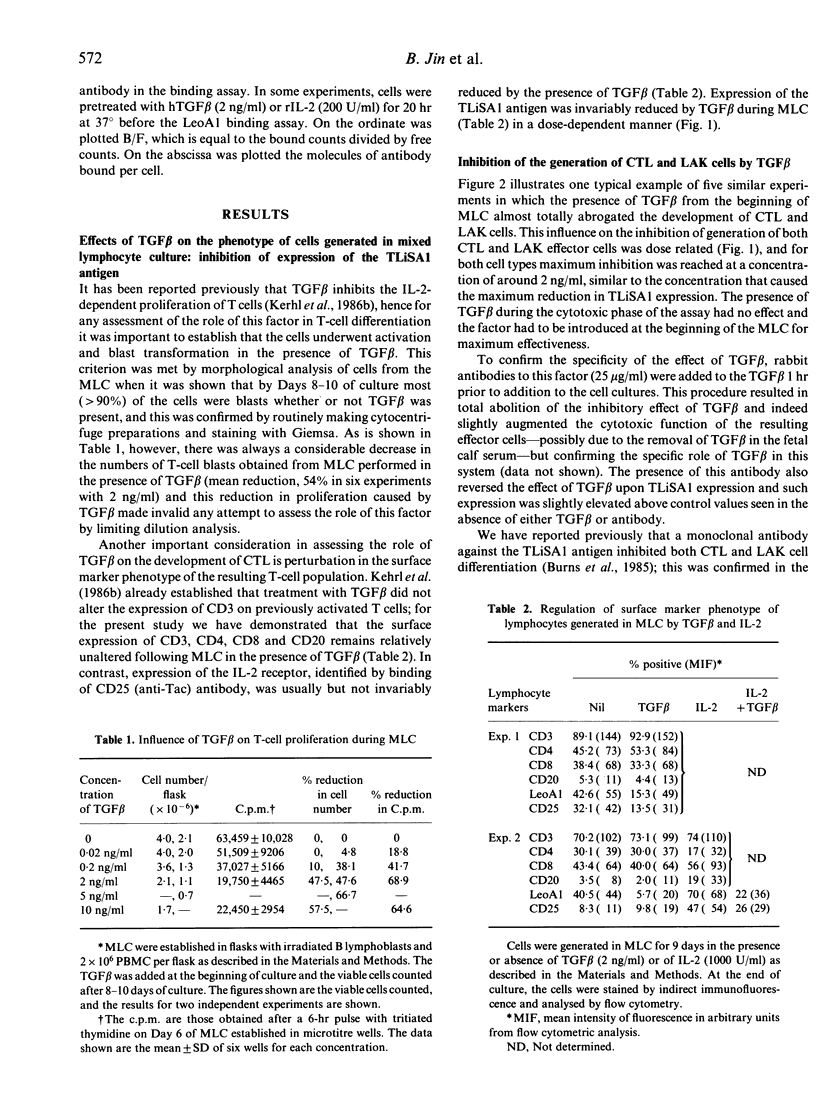
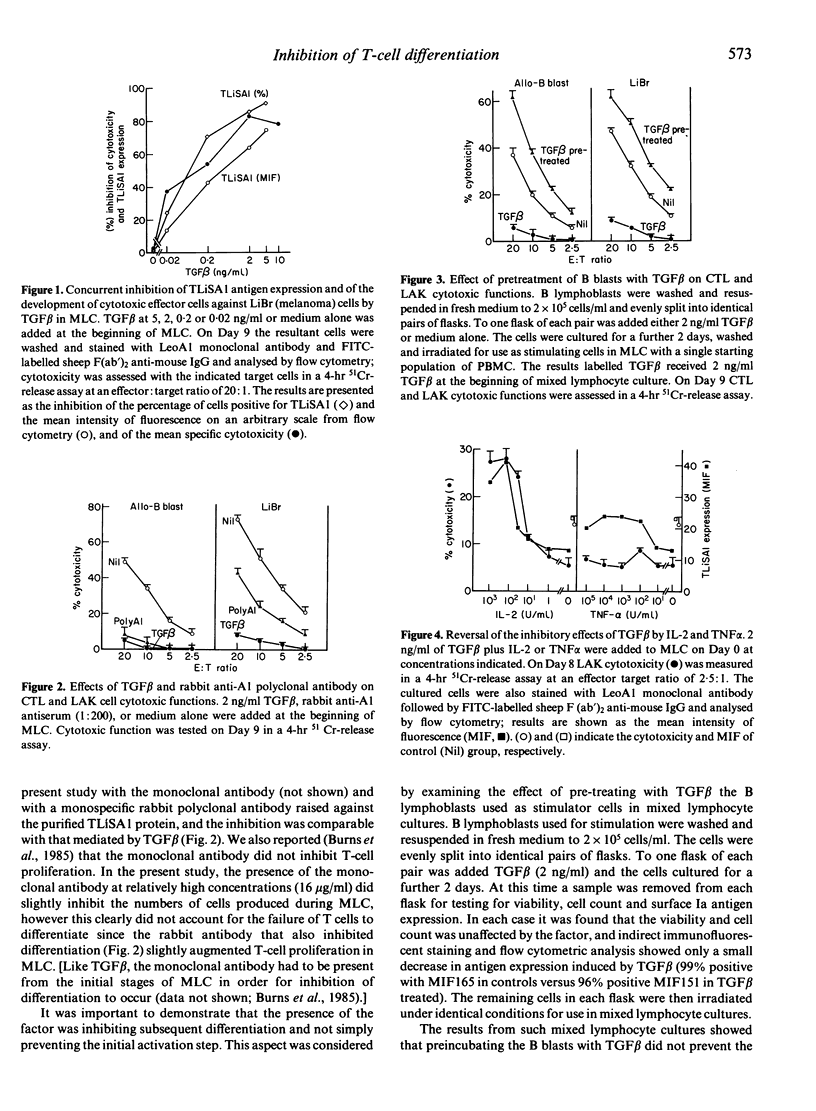
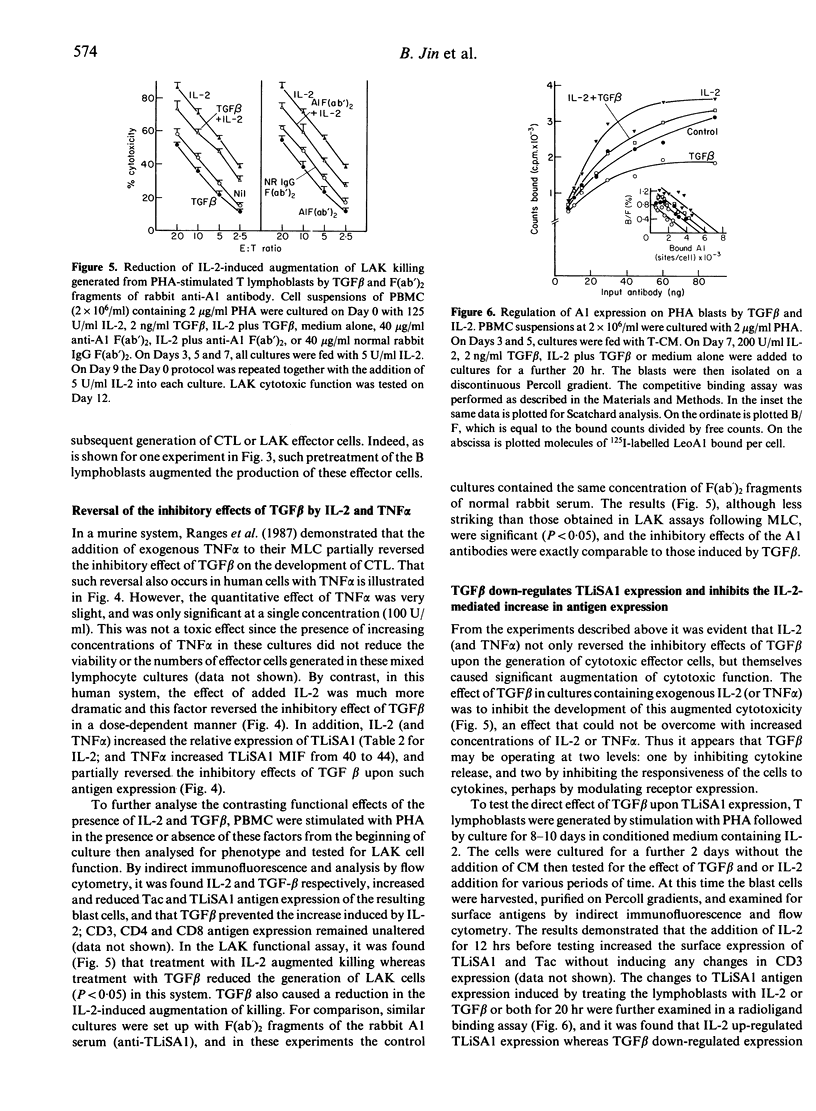
This study analysed the regulatory effects of transforming growth factor beta (TGF beta) on the expression of a 70,000 MW cell surface activation antigen, TLiSA1, involved in the differentiation of cytotoxic T lymphocytes (CTL) and lymphokine-activated killer (LAK) cells from their precursor(s), and also examined the role of TGF beta in the generation of these functional cells. TGF beta was shown to suppress the expression of TLiSA1 and to inhibit, in a dose-dependent manner, the generation of both CTL and LAK cells when present from the beginning of mixed lymphocyte culture; the same inhibitory effect upon the development of cytotoxic effector cells was observed with a monoclonal antibody and with monospecific rabbit antibodies against the TLiSA1 protein. Antibody to TGF beta reversed the inhibitory effect of the cytokine on differentiation and on TLiSA1 expression. Exogenous IL-2 or, to a lesser extent, tumour necrosis factor alpha (TNF alpha) added to mixed lymphocyte cultures (MLC) augmented both TLiSA1 antigen expression and cytotoxic function by the resulting blast cells; the co-addition of TGF beta inhibited both of these cytokine-mediated effects. Similarly, it was shown that phytohaemagglutini (PHA)-induced lymphoblasts up-regulate their surface expression of TLiSA1 and exhibit increased LAK activity in response to IL-2, and TGF beta inhibited both of these events; this IL-2-induced increase in LAK cell function was also inhibited by antibodies to TLiSA1. It is suggested that TLiSA1 antigen expression is intimately linked to the differentiation of cytotoxic effector cells and that such differentiation may be a distinct process from IL-2-induced proliferation, although both events can be regulated by TGF-beta.
Full text
PDF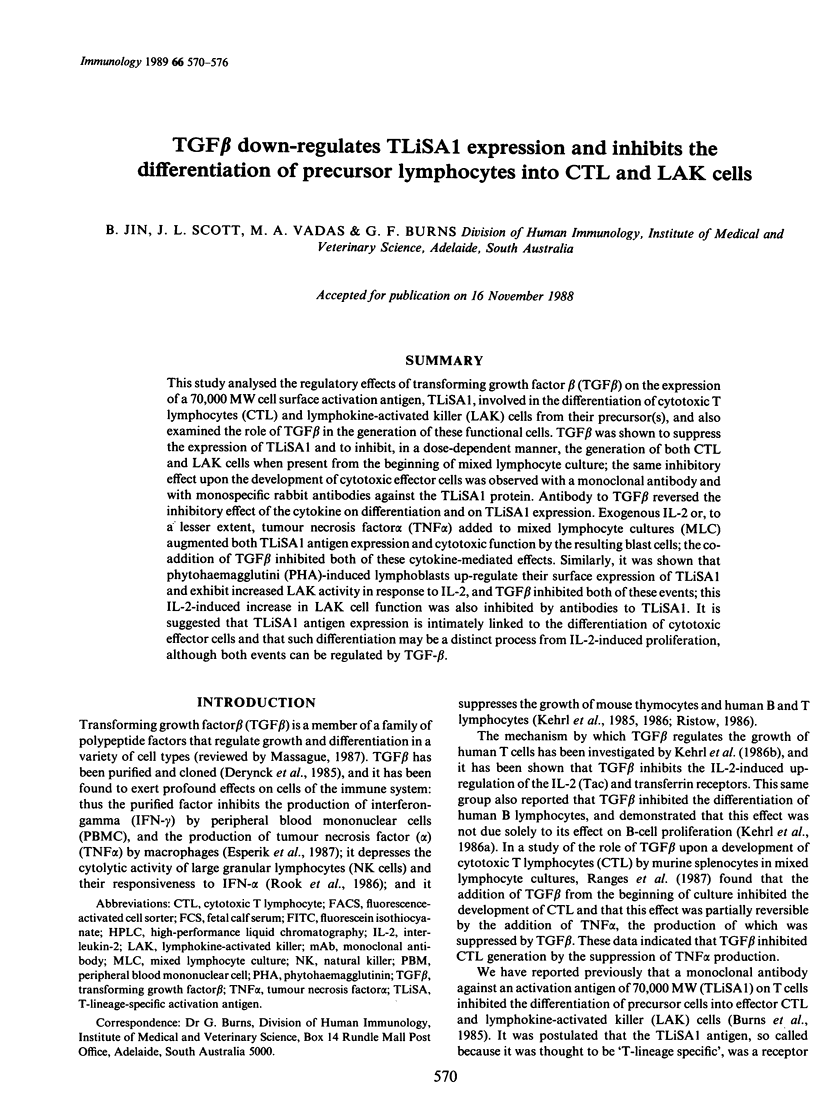



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomhoff H. K., Smeland E., Mustafa A. S., Godal T., Ohlsson R. Epstein-Barr virus mediates a switch in responsiveness to transforming growth factor, type beta, in cells of the B cell lineage. Eur J Immunol. 1987 Feb;17(2):299–301. doi: 10.1002/eji.1830170224. [DOI] [PubMed] [Google Scholar]
- Burns G. F., Boyd A. W., Beverley P. C. Two monoclonal anti-human T lymphocyte antibodies have similar biologic effects and recognize the same cell surface antigen. J Immunol. 1982 Oct;129(4):1451–1457. [PubMed] [Google Scholar]
- Burns G. F., Triglia T., Werkmeister J. A. In vitro generation of human activated lymphocyte killer cells: separate precursors and modes of generation of NK-like cells and "anomalous" killer cells. J Immunol. 1984 Sep;133(3):1656–1663. [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V., Bradley E. C. Interleukin 2-activated human killer cells are derived from phenotypically heterogeneous precursors. J Immunol. 1986 Nov 1;137(9):2814–2822. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Kumagai K., Balch C. M. Leu-11+ lymphocytes with natural killer (NK) activity are precursors of recombinant interleukin 2 (rIL 2)-induced activated killer (AK) cells. J Immunol. 1985 Feb;134(2):802–807. [PubMed] [Google Scholar]
- Kaufmann Y., Levanon M., Davidsohn J., Ramot B. Interleukin 2 induces human acute lymphocytic leukemia cells to manifest lymphokine-activated-killer (LAK) cytotoxicity. J Immunol. 1987 Aug 1;139(3):977–982. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A., Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986 Oct 1;164(4):1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranges G. E., Figari I. S., Espevik T., Palladino M. A., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow H. J. BSC-1 growth inhibitor/type beta transforming growth factor is a strong inhibitor of thymocyte proliferation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5531–5533. doi: 10.1073/pnas.83.15.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Rook A. H., Kehrl J. H., Wakefield L. M., Roberts A. B., Sporn M. B., Burlington D. B., Lane H. C., Fauci A. S. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986 May 15;136(10):3916–3920. [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Triglia T., Burns G. F. A method for in vitro clearance of mycoplasma from human cell lines. J Immunol Methods. 1983 Nov 11;64(1-2):133–139. doi: 10.1016/0022-1759(83)90391-5. [DOI] [PubMed] [Google Scholar]
- Watson D. B., Burns G. F., Mackay I. R. In vitro growth of B lymphocytes infiltrating human melanoma tissue by transformation with EBV: evidence for secretion of anti-melanoma antibodies by some transformed cells. J Immunol. 1983 May;130(5):2442–2447. [PubMed] [Google Scholar]


