Abstract
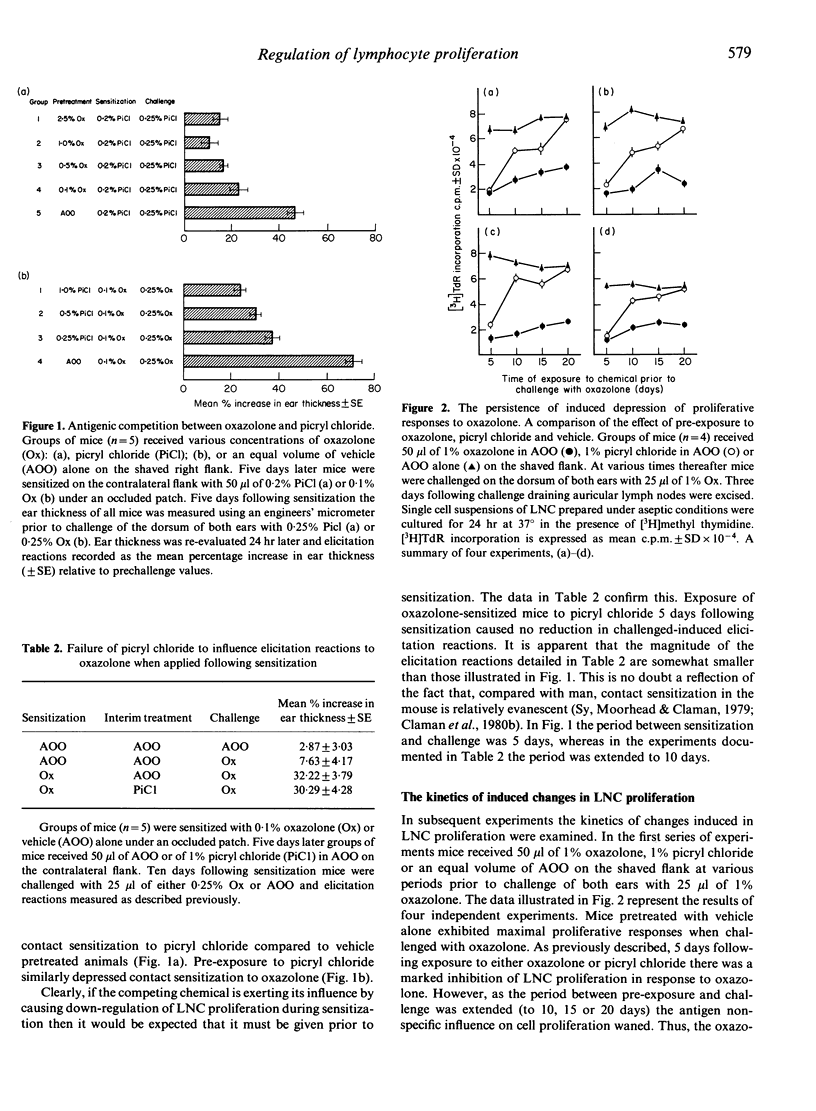
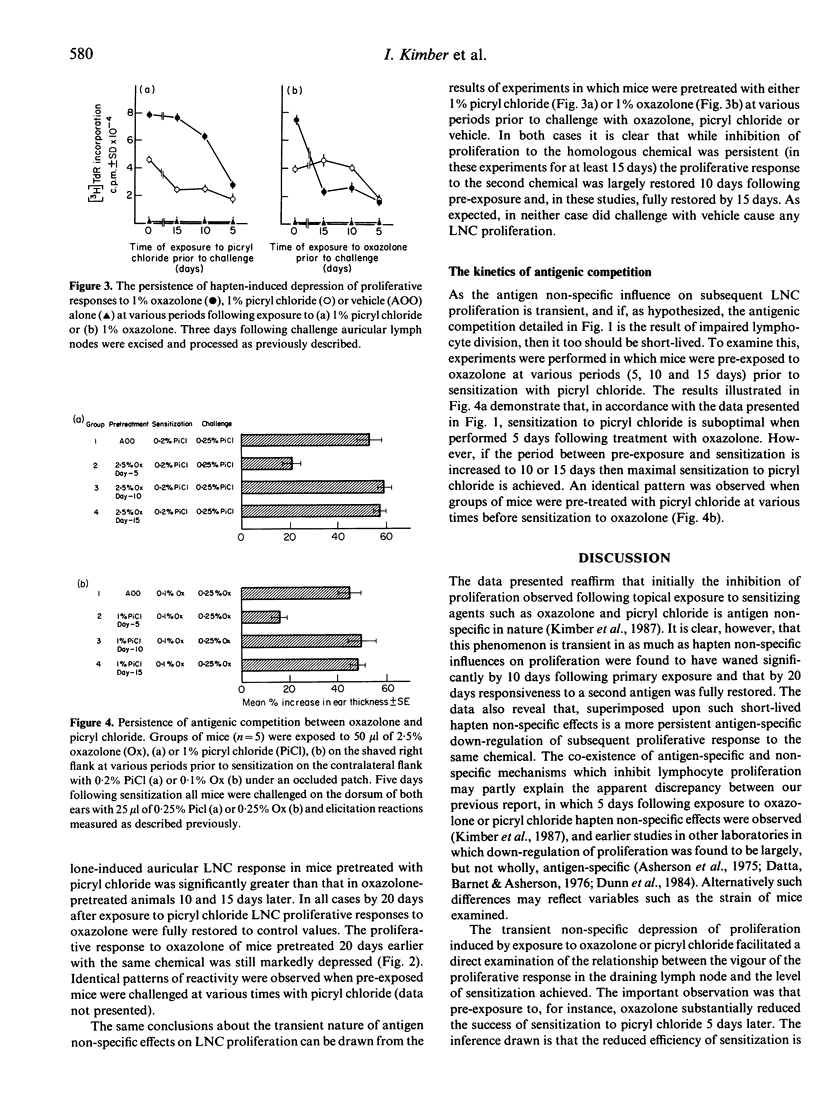
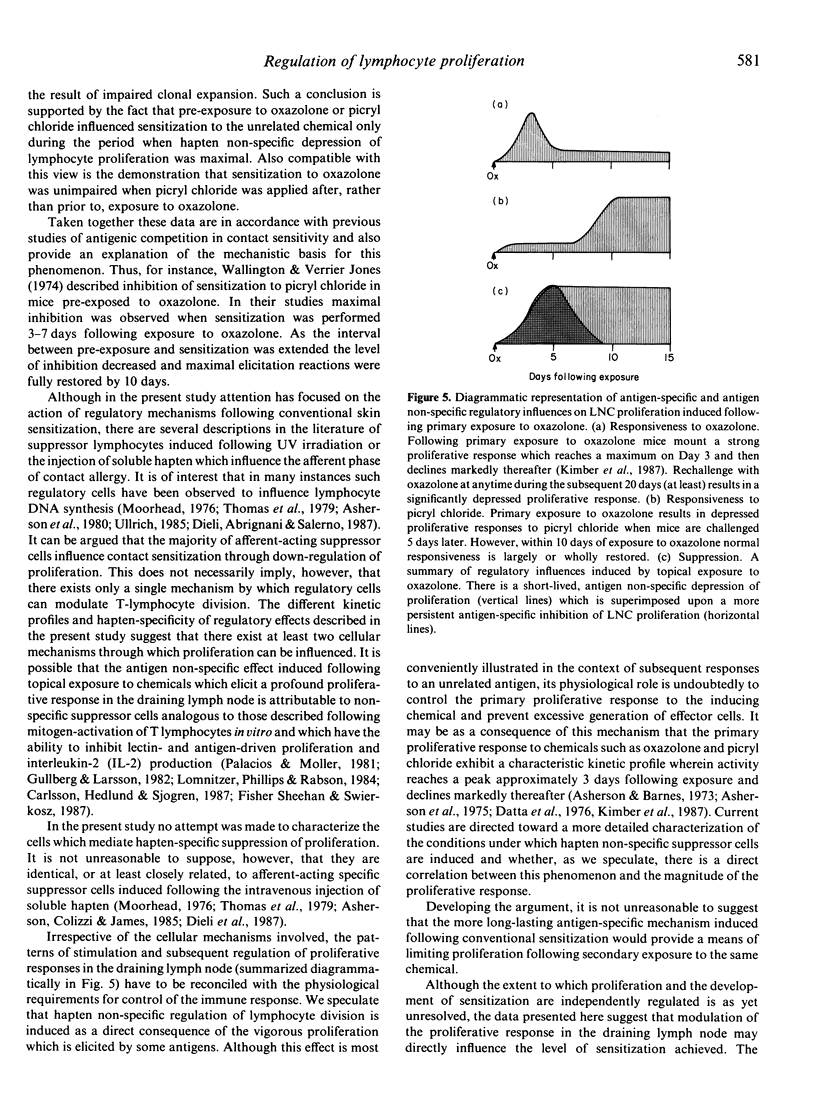
Epicutaneous exposure of mice to the contact sensitizing chemicals 4-ethoxymethylene-2-phenyl-oxazol-5-one (oxazolone) and 2,4,6-trinitrochlorobenzene (picryl chloride) causes an inhibition of proliferative responses induced following subsequent topical challenge. The effects on lymphocyte proliferation comprise both transient antigen non-specific and more persistent hapten-specific mechanisms. Pretreatment of mice with one chemical 5 days prior to sensitization with a second, at which time antigen non-specific influences on proliferative responses are manifest, results in depression of contact sensitization as measured by changes in ear thickness following challenge. If, however, the period between pretreatment and sensitization is extended the inhibition of contact sensitization disappears in parallel with a decline in the antigen non-specific depression of lymph node cell proliferation. These data reveal that there exist two homeostatic mechanisms which control proliferation in response to challenge with at least some antigens, and that the extent of lymphocyte proliferation directly influences the degree of contact sensitization achieved. Moreover these results demonstrate that, in some instances at least, competition between antigens may be a function of immunoregulatory influences on lymphocyte proliferation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Barnes R. M. Contact sensitivity in the mouse. XII. The use of DNA synthesis in vivo to determine the anatomical location of immunological unresponsiveness to picryl chloride. Immunology. 1973 Sep;25(3):495–508. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Colizzi V., James B. M. The control of the contact sensitivity skin reaction: T-suppressor afferent cell blocks the production of antigen-specific T-helper factor. Immunology. 1985 Mar;54(3):521–526. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Wood P. J., Mayhew B. Control of the immune response. I. Depression of DNA synthesis by immune lymph node cells. Immunology. 1975 Dec;29(6):1057–1065. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Perera M. A., Mayhew B., Thomas W. R. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the peyer's patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1977 Sep;33(1):145–155. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M. Suppression of contact sensitivity by T cells in the mouse. I. Demonstration that suppressor cells act on the effector stage of contact sensitivity; and their induction following in vitro exposure to antigen. Proc R Soc Lond B Biol Sci. 1974 Nov 5;187(1088):329–348. doi: 10.1098/rspb.1974.0078. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Hedlund G., Sjögren H. O. Abrogation of staphylococcal enterotoxin A-induced suppressor cell activity by the anti-Tac monoclonal antibody. Scand J Immunol. 1987 Jan;25(1):11–19. doi: 10.1111/j.1365-3083.1987.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Miller S. D., Conlon P. J., Moorhead J. W. Control of experimental contact sensitivity. Adv Immunol. 1980;30:121–157. doi: 10.1016/s0065-2776(08)60195-9. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Miller S. D., Sy M. S., Moorhead J. W. Suppressive mechanisms involving sensitization and tolerance in contact allergy. Immunol Rev. 1980;50:105–132. doi: 10.1111/j.1600-065x.1980.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Datta U., Barnet K., Asherson G. L. DNA synthesis in vitro by cells from mice immunized with picryl chloride: effect of injection of immune cells. Int Arch Allergy Appl Immunol. 1976;50(5):574–582. doi: 10.1159/000231561. [DOI] [PubMed] [Google Scholar]
- Dieli F., Abrignani S., Salerno A. T suppressor afferent cells which regulate contact sensitivity to picryl chloride act across genetic barrier. Immunol Lett. 1986 Nov 17;14(1):49–52. doi: 10.1016/0165-2478(86)90019-2. [DOI] [PubMed] [Google Scholar]
- Dunn I. S., Liberato D. J., Castagnoli N., Byers V. S. Induction of suppressor T cells for lymph node cell proliferation after contact sensitization of mice with a poison oak urushiol component. Immunology. 1984 Apr;51(4):773–781. [PMC free article] [PubMed] [Google Scholar]
- Elmets C. A., Bergstresser P. R., Tigelaar R. E., Wood P. J., Streilein J. W. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp Med. 1983 Sep 1;158(3):781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg M., Larsson E. L. Studies on induction and effector functions of concanavalin A-induced suppressor cells that limit TCGF production. J Immunol. 1982 Feb;128(2):746–750. [PubMed] [Google Scholar]
- Kimber I., Pierce B. B., Mitchell J. A., Kinnaird A. Depression of lymph node cell proliferation induced by oxazolone. Int Arch Allergy Appl Immunol. 1987;84(3):256–262. doi: 10.1159/000234432. [DOI] [PubMed] [Google Scholar]
- Lomnitzer R., Phillips R., Rabson A. R. Suppression of interleukin-2 production by human concanavalin A-induced suppressor cells. Cell Immunol. 1984 Jul;86(2):362–370. doi: 10.1016/0008-8749(84)90391-5. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Claman H. N. The induction of hapten-specific T cell tolerance by using hapten-modified lymphoid cells. I. Characteristics of tolerance induction. J Immunol. 1976 Nov;117(5 Pt 1):1519–1526. [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. Suppressor T cell mechanisms in contact sensitivity. I. Efferent blockade by syninduced suppressor T cells. J Immunol. 1978 Jul;121(1):265–273. [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFB in mice. VI. Inhibition of afferent sensitivity by suppressor T cells in adoptive tolerance. J Immunol. 1976 Sep;117(3):802–806. [PubMed] [Google Scholar]
- Noonan F. P., De Fabo E. C., Kripke M. L. Suppression of contact hypersensitivity by UV radiation and its relationship to UV-induced suppression of tumor immunity. Photochem Photobiol. 1981 Dec;34(6):683–689. [PubMed] [Google Scholar]
- Palacios R., Möller G. T cell growth factor abrogates concanavalin A-induced suppressor cell function. J Exp Med. 1981 May 1;153(5):1360–1365. doi: 10.1084/jem.153.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K. C., Swierkosz J. E. Functional analysis of antigen-nonspecific T-cell suppression. I. Effect of mitogen-induced T suppressor cells on helper-T-cell clones. Cell Immunol. 1987 Sep;108(2):269–282. doi: 10.1016/0008-8749(87)90212-7. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Moorhead J. W., Claman H. N. Regulation of cell mediated immunity by antibodies: possible role of anti-receptor antibodies in the regulation of contact sensitivity to DNFB in mice. J Immunol. 1979 Dec;123(6):2593–2598. [PubMed] [Google Scholar]
- Thomas W. R., Watkins M. C., Asherson G. L. Suppressor cells for the afferent phase of contact sensitivity to picryl chloride: inhibition of DNA synthesis induced by T cells from mice injected with picryl sulfonic acid. J Immunol. 1979 Jun;122(6):2300–2303. [PubMed] [Google Scholar]
- Ullrich S. E. Suppression of lymphoproliferation by hapten-specific suppressor T lymphocytes from mice exposed to ultraviolet radiation. Immunology. 1985 Feb;54(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Wallington T. B., Jones J. V. Competition between skin-sensitizing chemicals in the mouse. Immunology. 1974 Jul;27(1):125–131. [PMC free article] [PubMed] [Google Scholar]


