Abstract
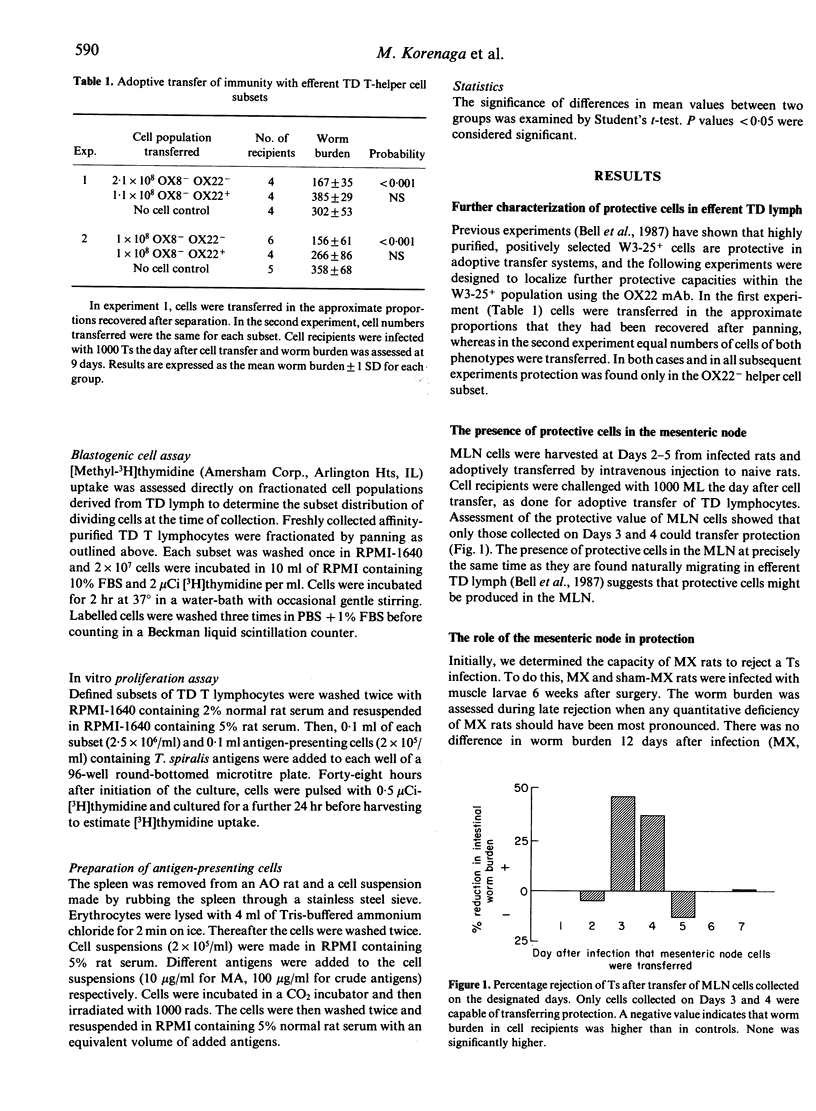
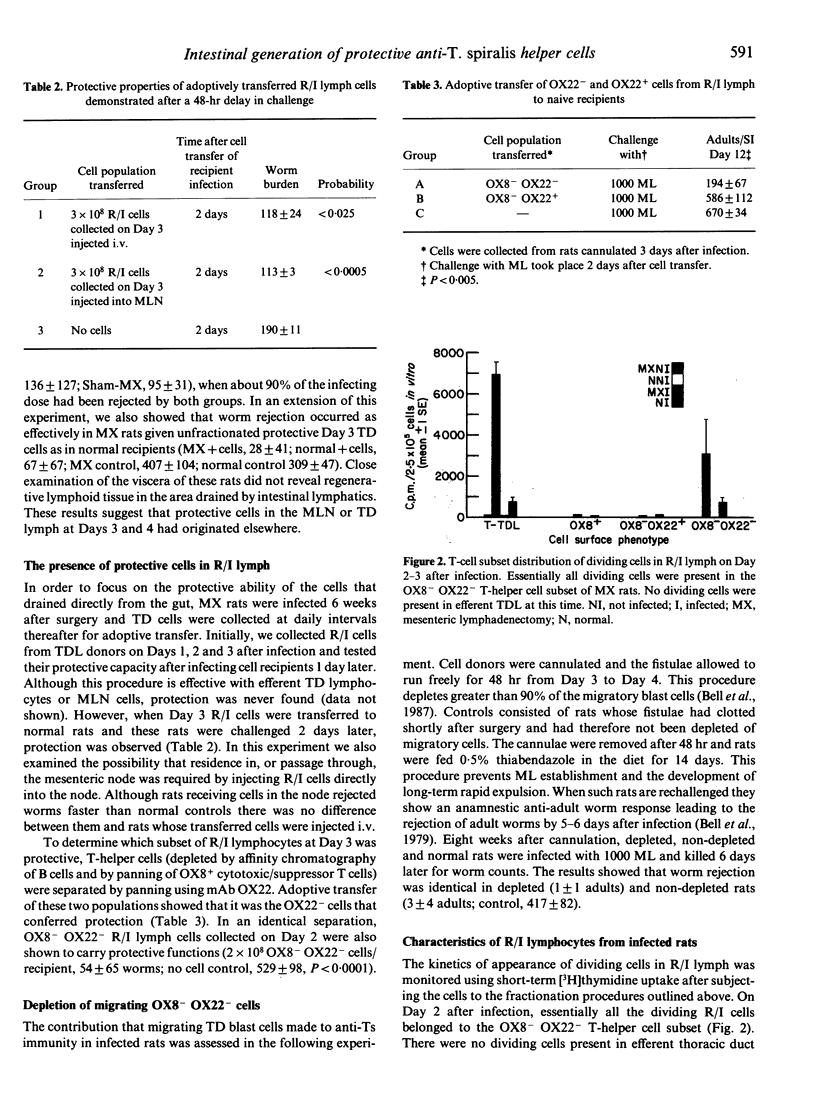
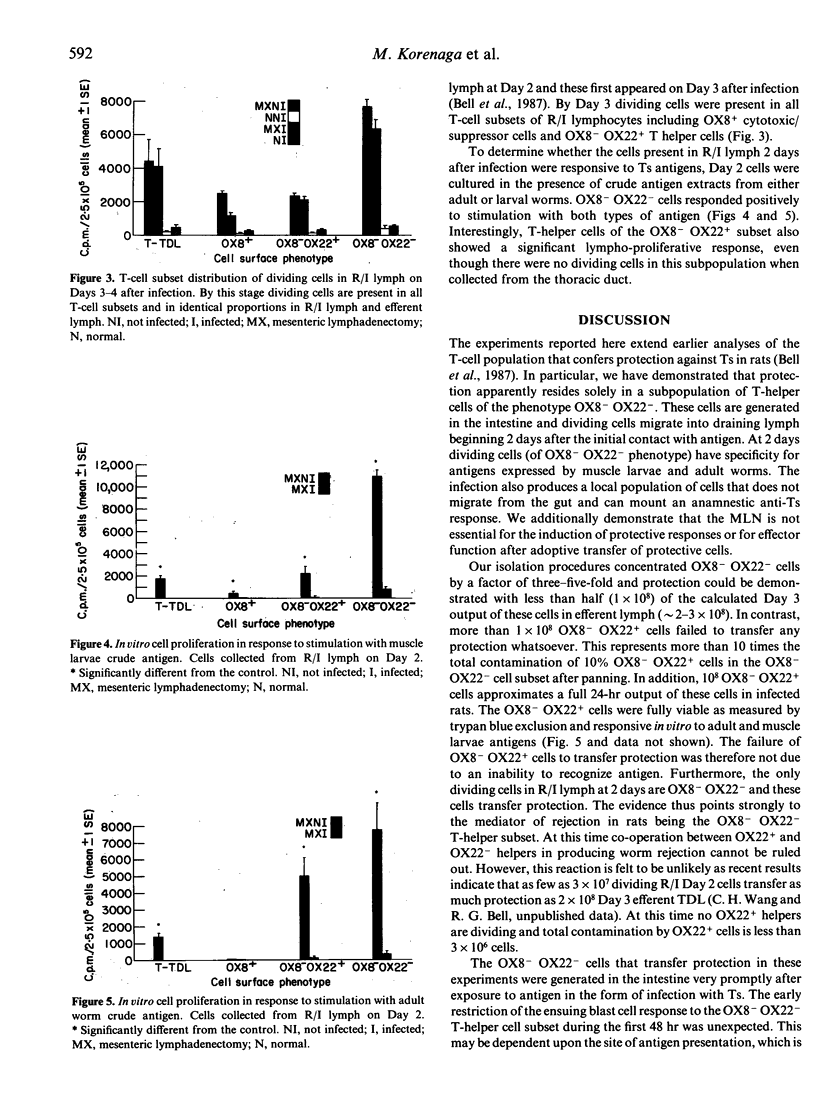
The phenotype of T-helper cells conferring protection against Trichinella spiralis (Ts) was studied using adoptive transfer procedures and T-helper cell subsets isolated by monoclonal antibodies. With these techniques OX8- OX22+ and OX8- OX22- T-helper cell populations were isolated from thoracic duct lymph (TDL) of infected rats three-five-fold more concentrated than in unfractionated lymph. The OX8- OX22- cell subset alone transferred enhanced rejection of adult worms from the intestine. The origin of protective OX8- OX22- cells was examined in mesenteric lymphadenectomized (MX) rats. After MX, protective cells were found in the cell population draining directly from the intestine on Days 2-3 after infection. Protective cells first appeared in the mesenteric lymph node (MLN) and efferent lymph at Day 3. MX rats rejected T. spiralis at the same time as intact controls and showed enhanced rejection when immune TDL were transfused. No evidence was found for a direct role of the MLN in the generation or expression of parasite rejection. Depletion of migrating OX8- OX22- blast cells by 48-hr drainage of TDL did not influence the expression of an anamnestic response to challenge infection. This suggests that an intestinally resident cell population has a substantial role in mediating primary worm rejection and anamnestic immunity. Day 2 OX8- OX22- cells from MX rats proliferated in response to the presentation of adult and muscle larvae antigens in vitro. We conclude that protection resides in the OX8- OX22- T-helper cell subset that is produced and functions in the intestine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. G., Korenaga M., Wang C. H. Characterization of a cell population in thoracic duct lymph that adoptively transfers rejection of adult Trichinella spiralis to normal rats. Immunology. 1987 Jun;61(2):221–227. [PMC free article] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D., Adams L. S. Trichinella spiralis: characterization and strain distribution of rapid expulsion in inbred mice. Exp Parasitol. 1982 Jun;53(3):301–314. doi: 10.1016/0014-4894(82)90073-x. [DOI] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D., Despommier D. D. Trichinella spiralis: mediation of the intestinal component of protective immunity in the rat by multiple, phase-specific, antiparasitic responses. Exp Parasitol. 1979 Apr;47(2):140–157. doi: 10.1016/0014-4894(79)90068-7. [DOI] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D., Woan M. C., Adams L. S. Trichinella spiralis: selective intestinal immune deviation in the rat. Exp Parasitol. 1983 Aug;56(1):129–142. doi: 10.1016/0014-4894(83)90104-2. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum E. D., Despommier D. D., McGregor D. D. Immunity to Trichinella spiralis. I. Transfer of resistance by two classes of lymphocytes. Immunology. 1977 Dec;33(6):787–795. [PMC free article] [PubMed] [Google Scholar]
- Crum E. D., McGregor D. D. Functional properties of T and B cells isolated by affinity chromatography from rat thoracic duct lymph. Cell Immunol. 1976 May;23(2):211–222. doi: 10.1016/0008-8749(76)90187-8. [DOI] [PubMed] [Google Scholar]
- Dessein A. J., Parker W. L., James S. L., David J. R. IgE antibody and resistance to infection. I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. J Exp Med. 1981 Feb 1;153(2):423–436. doi: 10.1084/jem.153.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grencis R. K., Riedlinger J., Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology. 1985 Oct;56(2):213–218. [PMC free article] [PubMed] [Google Scholar]
- Grencis R. K., Wakelin D. Short lived, dividing cells mediate adoptive transfer of immunity to Trichinella spiralis in mice. I. Availability of cells in primary and secondary infections in relation to cellular changes in the mesenteric lymph node. Immunology. 1982 Jun;46(2):443–450. [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Fisher R. IgA-containing plasma cells in the lamina propria of the gut: failure of a thoracic duct fistula to deplete the numbers in rat small intestine. Eur J Immunol. 1979 Jan;9(1):85–91. doi: 10.1002/eji.1830090118. [DOI] [PubMed] [Google Scholar]
- McWilliams M., Phillips-Quagliata J. M., Lamm M. E. Mesenteric lymph node B lymphoblasts which home to the small intestine are precommitted to IgA synthesis. J Exp Med. 1977 Apr 1;145(4):866–875. doi: 10.1084/jem.145.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Phillips S. M., Walker D., Abdel-Hafez S. K., Linette G. P., Doughty B. L., Perrin P. J., el Fathelbab N. The immune response to Schistosoma mansoni infections in inbred rats. VI. Regulation by T cell subpopulations. J Immunol. 1987 Oct 15;139(8):2781–2787. [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer H. W. Study of acute localised inflammation of the gastrointestinal tract: the effluent lymph. Gut. 1981 Oct;22(10):827–835. doi: 10.1136/gut.22.10.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. Evidence for the involvement of a bone marrow-derived cell population in the immune expulsion of Trichinella spiralis. Parasitology. 1977 Jun;74(3):225–234. doi: 10.1017/s0031182000047855. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Wright K. A. Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol. 1979 Jun;65(3):441–445. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]



