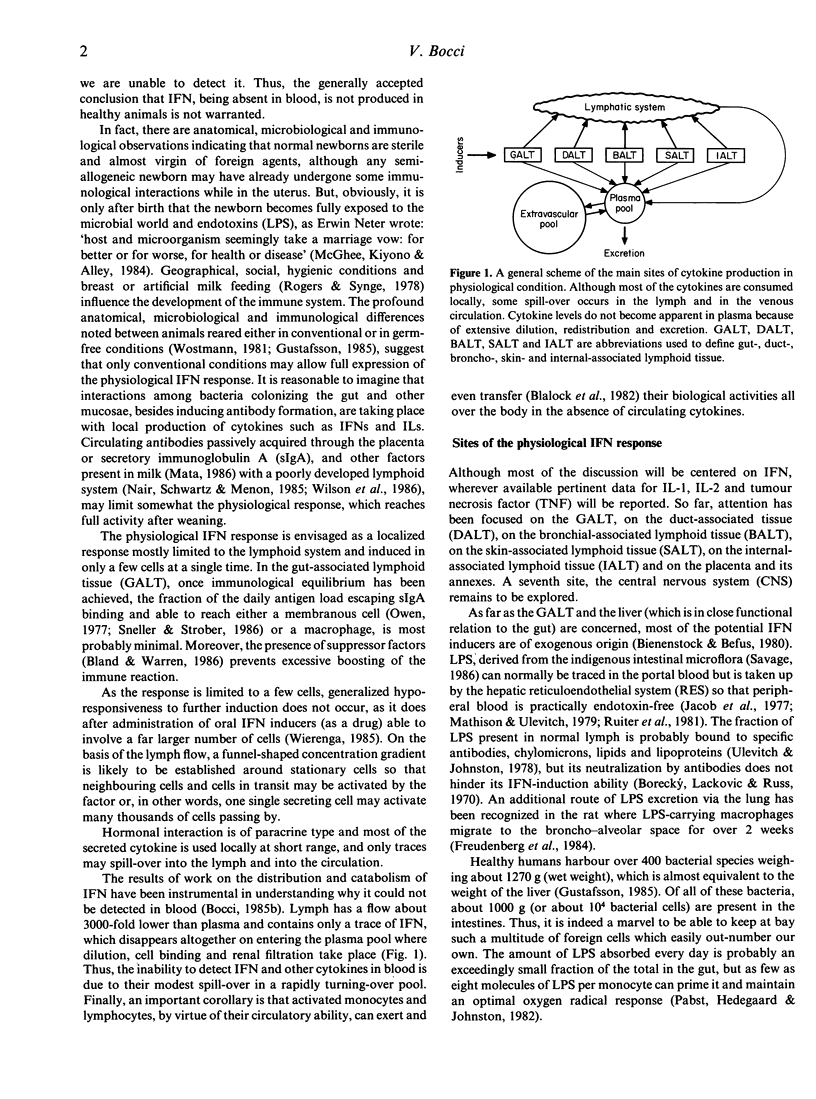
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton J. D., Myrvik Q. N. Production of interferon by alveolar macrophages. J Bacteriol. 1966 Jun;91(6):2300–2304. doi: 10.1128/jb.91.6.2300-2304.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartizal K. F., Salkowski C., Balish E. The influence of a gastrointestinal microflora on natural killer cell activity. J Reticuloendothel Soc. 1983 May;33(5):381–390. [PubMed] [Google Scholar]
- Belardelli F., Gessani S., Proietti E., Locardi C., Borghi P., Watanabe Y., Kawade Y., Gresser I. Studies on the expression of spontaneous and induced interferons in mouse peritoneal macrophages by means of monoclonal antibodies to mouse interferons. J Gen Virol. 1987 Aug;68(Pt 8):2203–2212. doi: 10.1099/0022-1317-68-8-2203. [DOI] [PubMed] [Google Scholar]
- Belardelli F., Vignaux F., Proietti E., Gresser I. Injection of mice with antibody to interferon renders peritoneal macrophages permissive for vesicular stomatitis virus and encephalomyocarditis virus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):602–606. doi: 10.1073/pnas.81.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Bever C. T., Jr, McFarlin D. E., Levy H. B. A comparison of interferon responses to poly ICLC in males and females. J Interferon Res. 1985 Summer;5(3):423–428. doi: 10.1089/jir.1985.5.423. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Blach-Olszewska Z., Cembrzýska-Nowak M. Synthesis of spontaneous interferon by mouse peritoneal cells in vitro. I. Attempts to elucidate the origin of spontaneous interferon. Acta Biol Med Ger. 1979;38(5-6):765–773. [PubMed] [Google Scholar]
- Blalock J. E., Baron S., Johnson H. M., Stanton G. J. Transmission of IFN-induced activities by cell to cell communication. Tex Rep Biol Med. 1981;41:344–349. [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E., 2nd Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J Immunol. 1986 Feb 1;136(3):963–970. [PubMed] [Google Scholar]
- Bocci V. Immunomodulators as local hormones: new insights regarding their clinical utilization. J Biol Response Mod. 1985 Aug;4(4):340–352. [PubMed] [Google Scholar]
- Bocci V. Is interferon produced in physiologic conditions? Med Hypotheses. 1980 Jul;6(7):735–745. doi: 10.1016/0306-9877(80)90091-2. [DOI] [PubMed] [Google Scholar]
- Bocci V. Metabolism of protein anticancer agents. Pharmacol Ther. 1987;34(1):1–49. doi: 10.1016/0163-7258(87)90090-8. [DOI] [PubMed] [Google Scholar]
- Bocci V., Muscettola M., Paulesu L., Grasso G. The physiological interferon response. II. Interferon is present in lymph but not in plasma of healthy rabbits. J Gen Virol. 1984 Jan;65(Pt 1):101–108. doi: 10.1099/0022-1317-65-1-101. [DOI] [PubMed] [Google Scholar]
- Bocci V., Muscettola M., Paulesu L., Grasso G. The physiological interferon response. V. Antiviral activity present in rat lymph is neutralized by anti-mouse interferon-gamma antibodies. Microbiologica. 1985 Oct;8(4):405–410. [PubMed] [Google Scholar]
- Bocci V., Paulesu L., Muscettola M., Vanni L. Presence of interferon in venous blood draining from gut-associated lymphoid tissue. Immunol Lett. 1986 Jan;12(1):25–29. doi: 10.1016/0165-2478(86)90076-3. [DOI] [PubMed] [Google Scholar]
- Bocci V., Paulesu L., Ricci M. G. The physiological interferon response: IV. Production of interferon by the perfused human placenta at term. Proc Soc Exp Biol Med. 1985 Oct;180(1):137–143. doi: 10.3181/00379727-180-42155. [DOI] [PubMed] [Google Scholar]
- Bocci V. Production and role of interferon in physiological conditions. Biol Rev Camb Philos Soc. 1981 Feb;56(1):49–85. doi: 10.1111/j.1469-185x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Bocci V. The role of sialic acid in determining the life-span of circulating cells and glycoproteins. Experientia. 1976 Feb 15;32(2):135–140. doi: 10.1007/BF01937727. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Lu L., Platzer E., Feit C., Juliano L., Rubin B. Y. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983 Sep;131(3):1300–1305. [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Lu L., Cooper S., Anderson S. L., Beyer G. S., Hoffman R., Rubin B. Y. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986 Jun 15;136(12):4487–4495. [PubMed] [Google Scholar]
- Cannon J. G., Dinarello C. A. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985 Mar 8;227(4691):1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Evans W. J., Hughes V. A., Meredith C. N., Dinarello C. A. Physiological mechanisms contributing to increased interleukin-1 secretion. J Appl Physiol (1985) 1986 Nov;61(5):1869–1874. doi: 10.1152/jappl.1986.61.5.1869. [DOI] [PubMed] [Google Scholar]
- Capobianchi M. R., Ameglio F., Tosi R., Dolei A. Differences in the expression and release of DR, BR, and DQ molecules in human cells treated with recombinant interferon gamma: comparison to other interferons. Hum Immunol. 1985 May;13(1):1–11. doi: 10.1016/0198-8859(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Cesario T., Goldstein A., Lindsey M., Dumars K., Tilles J. Antiviral activities of amniotic fluid. Proc Soc Exp Biol Med. 1981 Dec;168(3):403–407. doi: 10.3181/00379727-168-41295. [DOI] [PubMed] [Google Scholar]
- Chard T., Craig P. H., Menabawey M., Lee C. Alpha interferon in human pregnancy. Br J Obstet Gynaecol. 1986 Nov;93(11):1145–1149. doi: 10.1111/j.1471-0528.1986.tb08635.x. [DOI] [PubMed] [Google Scholar]
- Chisholm M., Cartwright T. Interferon production in leukaemia. Br J Haematol. 1978 Sep;40(1):43–50. doi: 10.1111/j.1365-2141.1978.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe R. M., Huang K. Y. Interferon preparations enhance phagocytosis in vivo. Infect Immun. 1976 Apr;13(4):1250–1257. doi: 10.1128/iai.13.4.1250-1257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiding P., Staeheli P., Haller O. Interferon-induced protein Mx accumulates in nuclei of mouse cells expressing resistance to influenza viruses. Virology. 1985 Jan 15;140(1):192–196. doi: 10.1016/0042-6822(85)90460-x. [DOI] [PubMed] [Google Scholar]
- Duc-Goiran P., Robert-Galliot B., Lopez J., Chany C. Unusual apparently constitutive interferons and antagonists in human placental blood. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5010–5014. doi: 10.1073/pnas.82.15.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English L. S., Whitehurst M. The production of T-cell growth factor (TCGF) in vivo in sheep. Cell Immunol. 1984 May;85(2):364–372. doi: 10.1016/0008-8749(84)90250-8. [DOI] [PubMed] [Google Scholar]
- Evans W. J., Meredith C. N., Cannon J. G., Dinarello C. A., Frontera W. R., Hughes V. A., Jones B. H., Knuttgen H. G. Metabolic changes following eccentric exercise in trained and untrained men. J Appl Physiol (1985) 1986 Nov;61(5):1864–1868. doi: 10.1152/jappl.1986.61.5.1864. [DOI] [PubMed] [Google Scholar]
- Fischer D. G., Rubinstein M. Spontaneous production of interferon-gamma and acid-labile interferon-alpha by subpopulations of human mononuclear cells. Cell Immunol. 1983 Oct 15;81(2):426–434. doi: 10.1016/0008-8749(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Fowler A. K., Reed C. D., Giron D. J. Identification of an interferon in murine placentas. Nature. 1980 Jul 17;286(5770):266–267. doi: 10.1038/286266a0. [DOI] [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocytes of the brain synthesize interleukin 3-like factors. J Immunol. 1985 Dec;135(6):4044–4047. [PubMed] [Google Scholar]
- Freudenberg N., Freudenberg M. A., Guzman J., Mittermayer C., Bandara K., Galanos C. Identification of endotoxin-positive cells in the rat lung during shock. Virchows Arch A Pathol Anat Histopathol. 1984;404(2):197–211. doi: 10.1007/BF00704064. [DOI] [PubMed] [Google Scholar]
- GORDON H. A., WOSTMANN B. S. Morphological studies on the germfree albino rat. Anat Rec. 1960 May;137:65–70. doi: 10.1002/ar.1091370108. [DOI] [PubMed] [Google Scholar]
- Galabru J., Robert N., Buffet-Janvresse C., Rivière Y., Hovanessian A. G. Continuous production of interferon in normal mice: effect of anti-interferon globulin, sex, age, strain and environment on the levels of 2-5A synthetase and p67K kinase. J Gen Virol. 1985 Apr;66(Pt 4):711–718. doi: 10.1099/0022-1317-66-4-711. [DOI] [PubMed] [Google Scholar]
- Gessani S., Belardelli F., Borghi P., Boraschi D., Gresser I. Correlation between the lipopolysaccharide response of mice and the capacity of mouse peritoneal cells to transfer an antiviral state. Role of endogenous interferon. J Immunol. 1987 Sep 15;139(6):1991–1998. [PubMed] [Google Scholar]
- Green J. A., Charette R. P., Yeh T. J., Smith C. B. Presence of interferon in acute- and convalescent-phase sera of humans with influenza or influenza-like illness of undetermined etiology. J Infect Dis. 1982 Jun;145(6):837–841. doi: 10.1093/infdis/145.6.837. [DOI] [PubMed] [Google Scholar]
- Gresser I., Belardelli F., Maury C., Maunoury M. T., Tovey M. G. Injection of mice with antibody to interferon enhances the growth of transplantable murine tumors. J Exp Med. 1983 Dec 1;158(6):2095–2107. doi: 10.1084/jem.158.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Vignaux F., Belardelli F., Tovey M. G., Maunoury M. T. Injection of mice with antibody to mouse interferon alpha/beta decreases the level of 2'-5' oligoadenylate synthetase in peritoneal macrophages. J Virol. 1985 Jan;53(1):221–227. doi: 10.1128/jvi.53.1.221-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Gresser I., Lindenmann J. Genetically determined, interferon-dependent resistance to influenza virus in mice. J Exp Med. 1979 Mar 1;149(3):601–612. doi: 10.1084/jem.149.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C., Saurat J. H., Schmitt A., Jaunin F., Dayer J. M. Interleukin 1 is present in normal human epidermis. J Immunol. 1986 May 1;136(9):3317–3323. [PubMed] [Google Scholar]
- Hovanessian A. G., Rollin P., Rivière Y., Pouillart P., Sureau P., Montagnier L. Protein kinase in human plasma analogous to that present in control and interferon-treated HeLa cells. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1371–1377. doi: 10.1016/0006-291x(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Jacob A. I., Goldberg P. K., Bloom N., Degenshein G. A., Kozinn P. J. Endotoxin and bacteria in portal blood. Gastroenterology. 1977 Jun;72(6):1268–1270. [PubMed] [Google Scholar]
- Jameson P., Grossberg S. E. Production of interferon by human tumor cell lines. Arch Virol. 1979;62(3):209–219. doi: 10.1007/BF01317553. [DOI] [PubMed] [Google Scholar]
- Jelinek D. F., Splawski J. B., Lipsky P. E. The roles of interleukin 2 and interferon-gamma in human B cell activation, growth and differentiation. Eur J Immunol. 1986 Aug;16(8):925–932. doi: 10.1002/eji.1830160809. [DOI] [PubMed] [Google Scholar]
- Johnson S. R., Petzold C. R., Galask R. P. Qualitative and quantitative changes of the vaginal microbial flora during the menstrual cycle. Am J Reprod Immunol Microbiol. 1985 Sep;9(1):1–5. doi: 10.1111/j.1600-0897.1985.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H., Voss R. M., Good R. A. IL 1-like activities present in murine amniotic fluid. A significantly larger amount of IL 1 beta-like activity is present in the amniotic fluid of autoimmune NZB mice. J Immunol. 1987 May 15;138(10):3300–3307. [PubMed] [Google Scholar]
- Karnovsky M. L. Muramyl peptides in mammalian tissues and their effects at the cellular level. Fed Proc. 1986 Oct;45(11):2556–2560. [PubMed] [Google Scholar]
- Kirchner H., Weyland A., Storch E. Local interferon induction by bacterial lipopolysaccharide in mice after pretreatment with Corynebacterium parvum. J Interferon Res. 1986 Oct;6(5):483–487. doi: 10.1089/jir.1986.6.483. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Elias J. A., Kay S. L., Rossman M. D., Nowell P. C., Daniele R. P. Spontaneous production of interleukin-1 by human alveolar macrophages. Clin Immunol Immunopathol. 1983 Dec;29(3):443–450. doi: 10.1016/0090-1229(83)90047-8. [DOI] [PubMed] [Google Scholar]
- Le J., Lin J. X., Henriksen-DeStefano D., Vilcek J. Bacterial lipopolysaccharide-induced interferon-gamma production: roles of interleukin 1 and interleukin 2. J Immunol. 1986 Jun 15;136(12):4525–4530. [PubMed] [Google Scholar]
- Lebon P., Girard S., Thépot F., Chany C. The presence of alpha-interferon in human amniotic fluid. J Gen Virol. 1982 Apr;59(Pt 2):393–396. doi: 10.1099/0022-1317-59-2-393. [DOI] [PubMed] [Google Scholar]
- Lefkowitz E. J., Fleischmann W. R., Jr An inhibitor of interferon action: II. Biological properties of the IFN-gamma-associated inhibitor of interferon action. J Interferon Res. 1985 Winter;5(1):101–110. doi: 10.1089/jir.1985.5.101. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Playfair J. H. Serum IL-2 inhibitor in mice. I. Increase during infection. Immunology. 1985 Sep;56(1):113–118. [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Haimovitz A., Chen Y., Chan J., Rosenstreich D. L. Characterization of a human interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3882–3886. [PubMed] [Google Scholar]
- Mamus S. W., Beck-Schroeder S., Zanjani E. D. Suppression of normal human erythropoiesis by gamma interferon in vitro. Role of monocytes and T lymphocytes. J Clin Invest. 1985 May;75(5):1496–1503. doi: 10.1172/JCI111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Maza O., Andersson U., Andersson J., Britton S., De Ley M. Spontaneous production of interferon-gamma in adult and newborn humans. J Immunol. 1984 Jan;132(1):251–255. [PubMed] [Google Scholar]
- Masek K. Multiplicity of biological effects of muramyl dipeptide. Methods Find Exp Clin Pharmacol. 1986 Feb;8(2):97–99. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- McGhee J. R., Kiyono H., Alley C. D. Gut bacterial endotoxin: influence on gut-associated lymphoreticular tissue and host immune function. Surv Immunol Res. 1984;3(4):241–252. doi: 10.1007/BF02919039. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nair M. P., Schwartz S. A., Menon M. Association of decreased natural and antibody-dependent cellular cytotoxicity and production of natural killer cytotoxic factor and interferon in neonates. Cell Immunol. 1985 Aug;94(1):159–171. doi: 10.1016/0008-8749(85)90093-0. [DOI] [PubMed] [Google Scholar]
- Nair P. N., Schroeder H. E. Duct-associated lymphoid tissue (DALT) of minor salivary glands and mucosal immunity. Immunology. 1986 Feb;57(2):171–180. [PMC free article] [PubMed] [Google Scholar]
- Newmark P. Oncogenes and cell growth. Nature. 1987 May 14;327(6118):101–101. doi: 10.1038/327101a0. [DOI] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Palmblad J., Cantell K., Strander H., Fröberg J., Karlsson C. G., Levi L., Granström M., Unger P. Stressor exposure and immunological response in man: interferon-producing capacity and phagocytosis. J Psychosom Res. 1976;20(3):193–199. doi: 10.1016/0022-3999(76)90020-9. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Friedman R. M. Interferon-induced alterations in cells: relevance to viral and nonviral diseases. Lab Invest. 1983 Jul;49(1):4–18. [PubMed] [Google Scholar]
- Proietti E., Gessani S., Belardelli F., Gresser I. Mouse peritoneal cells confer an antiviral state on mouse cell monolayers: role of interferon. J Virol. 1986 Feb;57(2):456–463. doi: 10.1128/jvi.57.2.456-463.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley V. Psychoneuroendocrine influences on immunocompetence and neoplasia. Science. 1981 Jun 5;212(4499):1100–1109. doi: 10.1126/science.7233204. [DOI] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Ruiter D. J., van der Meulen J., Brouwer A., Hummel M. J., Mauw B. J., van der Ploeg J. C., Wisse E. Uptake by liver cells of endotoxin following its intravenous injection. Lab Invest. 1981 Jul;45(1):38–45. [PubMed] [Google Scholar]
- Savage D. C. Gastrointestinal microflora in mammalian nutrition. Annu Rev Nutr. 1986;6:155–178. doi: 10.1146/annurev.nu.06.070186.001103. [DOI] [PubMed] [Google Scholar]
- Schattner A., Wallach D., Merlin G., Hahn T., Levin S., Revel M. Assay of an interferon-induced enzyme in white blood cells as a diagnostic aid in viral diseases. Lancet. 1981 Sep 5;2(8245):497–500. doi: 10.1016/s0140-6736(81)90883-7. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., May L. T., Tamm I., Vilcek J. Human beta 2 interferon and B-cell differentiation factor BSF-2 are identical. Science. 1987 Feb 13;235(4790):731–732. doi: 10.1126/science.3492764. [DOI] [PubMed] [Google Scholar]
- Sergiescu D., Gerfaux J., Joret A. M., Chany C. Persistent expression of v-mos oncogene in transformed cells that revert to nonmalignancy after prolonged treatment with interferon. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5764–5768. doi: 10.1073/pnas.83.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Smith T. J., Wagner R. R. Rabbit macrophage interferons. I. Conditions for biosynthesis by virus-infected and uninfected cells. J Exp Med. 1967 Apr 1;125(4):559–577. doi: 10.1084/jem.125.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller M. C., Strober W. M cells and host defense. J Infect Dis. 1986 Nov;154(5):737–741. doi: 10.1093/infdis/154.5.737. [DOI] [PubMed] [Google Scholar]
- Spitz M., Gearing A., Callus M., Spitz L., Thorpe R. Interleukin-2 in vivo: production of and response to interleukin-2 in lymphoid organs undergoing a primary immune response to heterologous erythrocytes. Immunology. 1985 Mar;54(3):527–532. [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J. W. Lymphocyte traffic, T-cell malignancies and the skin. J Invest Dermatol. 1978 Sep;71(3):167–171. doi: 10.1111/1523-1747.ep12547071. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Matsuyama M., Fujii M., Kannagi M., Hinuma Y. Establishment of human cell lines constitutively producing immune interferon: transformation of normal T cells by a human retrovirus. J Immunol. 1983 Oct;131(4):1611–1612. [PubMed] [Google Scholar]
- Tedeschi B., Barrett J. N., Keane R. W. Astrocytes produce interferon that enhances the expression of H-2 antigens on a subpopulation of brain cells. J Cell Biol. 1986 Jun;102(6):2244–2253. doi: 10.1083/jcb.102.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey E. R., Chapman M. D., Platts-Mills T. A. Mite faeces are a major source of house dust allergens. Nature. 1981 Feb 12;289(5798):592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Streuli M., Gresser I., Gugenheim J., Blanchard B., Guymarho J., Vignaux F., Gigou M. Interferon messenger RNA is produced constitutively in the organs of normal individuals. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5038–5042. doi: 10.1073/pnas.84.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Kobayashi M., Rosen M., Loudon R., Murphy M., Perussia B. Tumor necrosis factor and lymphotoxin induce differentiation of human myeloid cell lines in synergy with immune interferon. J Exp Med. 1986 Oct 1;164(4):1206–1225. doi: 10.1084/jem.164.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viti A., Muscettola M., Paulesu L., Bocci V., Almi A. Effect of exercise on plasma interferon levels. J Appl Physiol (1985) 1985 Aug;59(2):426–428. doi: 10.1152/jappl.1985.59.2.426. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Fertsch D. Endogenous interferon production by endotoxin-responsive macrophages provides an autostimulatory differentiation signal. Infect Immun. 1984 Aug;45(2):417–423. doi: 10.1128/iai.45.2.417-423.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Fertsch D. Macrophages from endotoxin-hyporesponsive (Lpsd) C3H/HeJ mice are permissive for vesicular stomatitis virus because of reduced levels of endogenous interferon: possible mechanism for natural resistance to virus infection. J Virol. 1987 Mar;61(3):812–818. doi: 10.1128/jvi.61.3.812-818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Nagington J., Scott G. M., Secher D. S. An immunoradiometric assay of serum interferon using a monoclonal antibody. J Gen Virol. 1982 Sep;62(Pt 1):181–185. doi: 10.1099/0022-1317-62-1-181. [DOI] [PubMed] [Google Scholar]
- Wierenga W. Antiviral and other bioactivities of pyrimidinones. Pharmacol Ther. 1985;30(1):67–89. doi: 10.1016/0163-7258(85)90048-8. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Westall J., Johnston L., Lewis D. B., Dower S. K., Alpert A. R. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest. 1986 Mar;77(3):860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostmann B. S., Pleasants J. R., Bealmear P. Dietary stimulation of immune mechanisms. Fed Proc. 1971 Nov-Dec;30(6):1779–1784. [PubMed] [Google Scholar]
- Wostmann B. S. The germfree animal in nutritional studies. Annu Rev Nutr. 1981;1:257–279. doi: 10.1146/annurev.nu.01.070181.001353. [DOI] [PubMed] [Google Scholar]
- Yaar M., Palleroni A. V., Gilchrest B. A. Normal human epidermis contains an interferon-like protein. J Cell Biol. 1986 Oct;103(4):1349–1354. doi: 10.1083/jcb.103.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Engler H., Kirchner H. Experimental infection of inbred mice with herpes simplex virus. III. Comparison between newborn and adult C57BL/6 mice. J Gen Virol. 1982 May;60(Pt 1):25–29. doi: 10.1099/0022-1317-60-1-25. [DOI] [PubMed] [Google Scholar]
- Zoumbos N. C., Gascon P., Djeu J. Y., Young N. S. Interferon is a mediator of hematopoietic suppression in aplastic anemia in vitro and possibly in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):188–192. doi: 10.1073/pnas.82.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maeyer E., Fauve R. M., de Maeyer-Guignard J. Production d'interféron au niveau du macrophage. Ann Inst Pasteur (Paris) 1971 Mar;120(3):438–446. [PubMed] [Google Scholar]



