Abstract
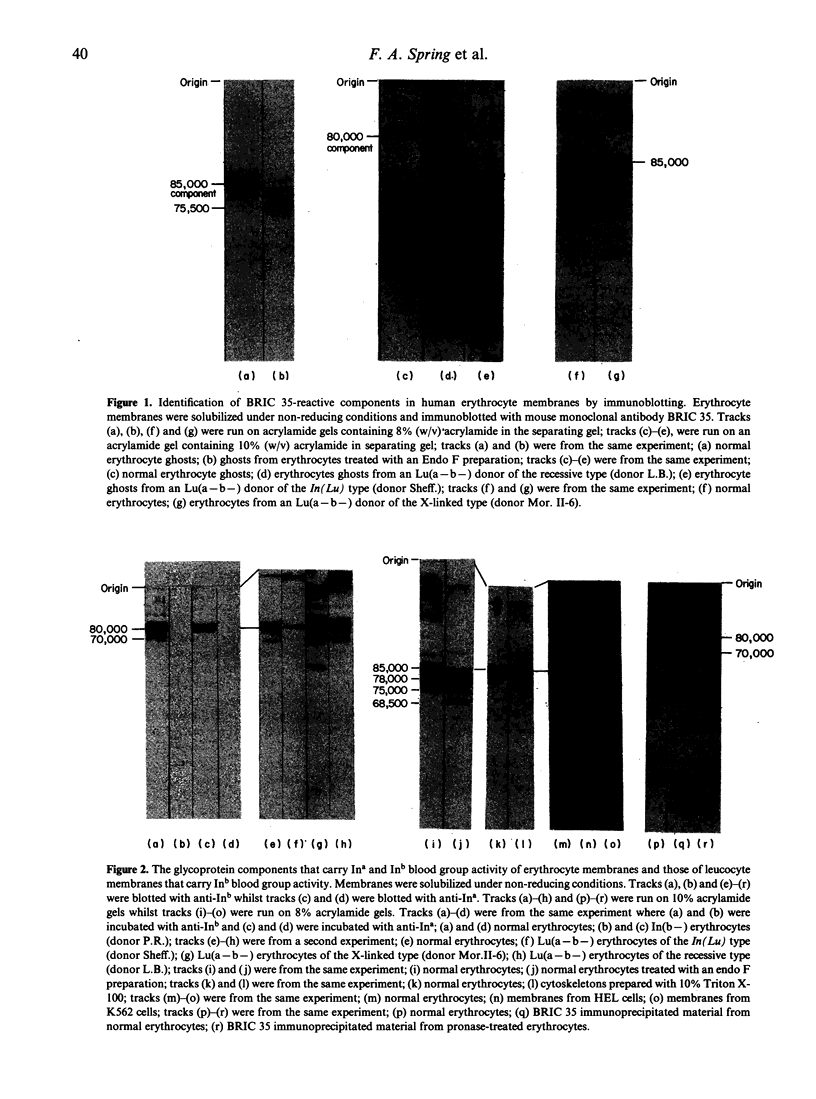
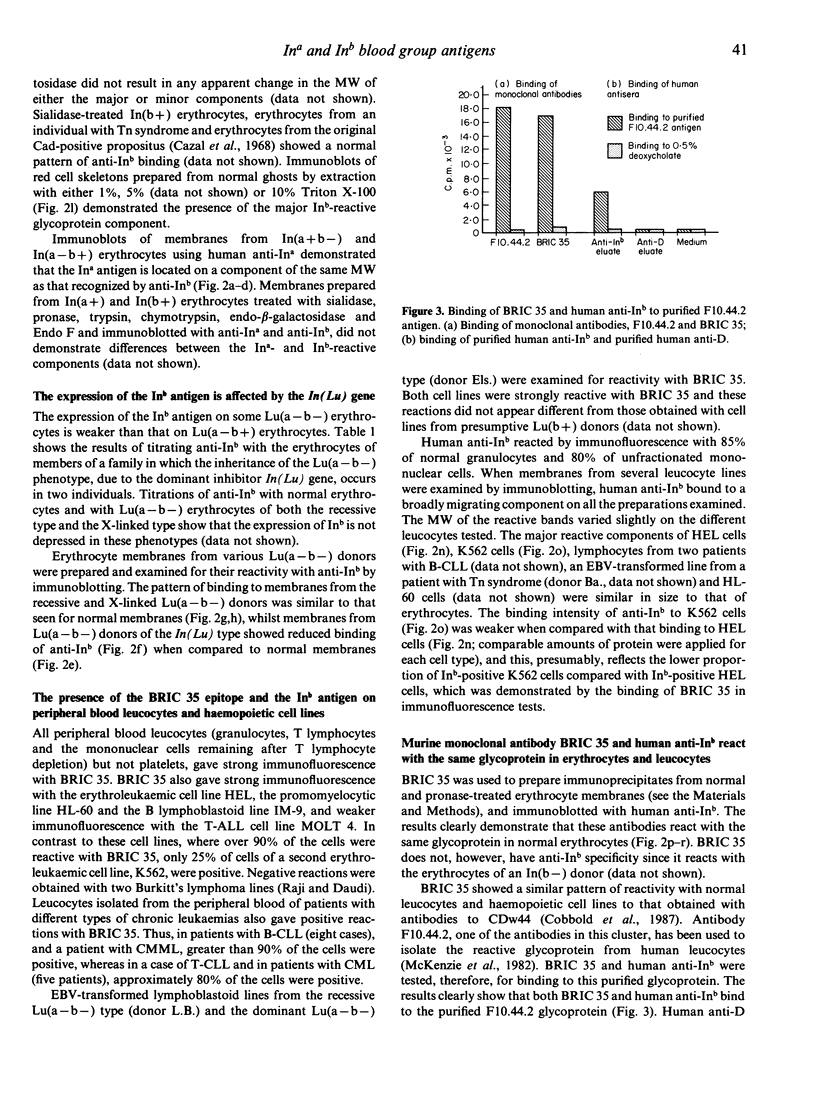
The Ina and Inb blood group antigens were found to be located on an erythrocyte membrane glycoprotein of 80,000 MW by immunoblotting with human anti-Ina and anti-Inb antibodies under non-reducing conditions. This glycoprotein is shown here to be identical to that defined by monoclonal antibodies to CDw44, and a new murine monoclonal antibody (BRIC 35) is added to this cluster. Experiments with endo-beta-galactosidase and Endo F preparations suggest that the glycoprotein contains one or more N-glycans but that these oligosaccharides do not contain extensive poly-N-acetyllactosaminyl sequences. Experiments using membranes prepared from sialidase-treated normal erythrocytes, from Tn erythrocytes and from Cad erythrocytes suggest that the glycoprotein does not contain a substantial content of O-glycans. The Inb antigen and the epitope defined by a murine monoclonal antibody (BRIC 35) show reduced expression on Lu(a-b-) erythrocytes which result from the effect of the dominant inhibitor gene In(Lu). Evidence is presented here that the Inb antigen is expressed on normal granulocytes and lymphocytes and on the haemopoietic cell lines HEL, K562 and HL-60, a lymphoblastoid cell line and lymphocytes from two patients with B-CLL.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Advani H., Zamor J., Judd W. J., Johnson C. L., Marsh W. L. Inactivation of Kell blood group antigens by 2-aminoethylisothiouronium bromide. Br J Haematol. 1982 May;51(1):107–115. doi: 10.1111/j.1365-2141.1982.tb07295.x. [DOI] [PubMed] [Google Scholar]
- Badakere S. S., Parab B. B., Bhatia H. M. Further observations on the Ina (Indian) antigen in Indian populations. Vox Sang. 1974;26(4):400–401. doi: 10.1111/j.1423-0410.1974.tb02713.x. [DOI] [PubMed] [Google Scholar]
- Branch D. R., Muensch H. A., Sy Siok Hian A. L., Petz L. D. Disulfide bonds are a requirement for Kell and Cartwright (Yta) blood group antigen integrity. Br J Haematol. 1983 Aug;54(4):573–578. doi: 10.1111/j.1365-2141.1983.tb02136.x. [DOI] [PubMed] [Google Scholar]
- Cazal P., Monis M., Caubel J., Brives J. Polyagglutinabilité héréditaire dominante: antigène privé (Cad) correspondant à un anticorps public et à une lectine de Dolichos biflorus. Rev Fr Transfus. 1968 Oct 3;11(3):209–221. doi: 10.1016/s0035-2977(68)80050-1. [DOI] [PubMed] [Google Scholar]
- Cruz T. F., Quackenbush E. J., Letarte M., Moscarello M. A. Effects of development and aging on the concentration of a human brain antigen. Neurosci Lett. 1985 Sep 6;59(3):253–257. doi: 10.1016/0304-3940(85)90140-5. [DOI] [PubMed] [Google Scholar]
- Cruz T. F., Quackenbush E. J., Letarte M., Moscarello M. A. Elevated levels of a glycoprotein antigen (P-80) in gray and white matter of brain from victims of multiple sclerosis. Neurochem Res. 1986 Jun;11(6):877–889. doi: 10.1007/BF00965211. [DOI] [PubMed] [Google Scholar]
- DARNBOROUGH J., FIRTH R., BILES C. M., GOLDSMITH K. L., CRAWFORD M. N. A "new" antibody anti-Lu-a-Lu-b and two further examples of the genotype Lu(a-b-). Nature. 1963 May 25;198:796–796. doi: 10.1038/198796a0. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. Demonstration with monoclonal antibodies of an unusual mononuclear cell infiltrate and loss of normal epithelial membrane antigens in human breast carcinomas. Lancet. 1981 Aug 29;2(8244):434–438. doi: 10.1016/s0140-6736(81)90773-x. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. The membrane antigens of human colorectal cancer cells: demonstration with monoclonal antibodies of heterogeneity within and between tumours and of anomalous expression of HLA-DR. Eur J Cancer Clin Oncol. 1983 Feb;19(2):209–220. doi: 10.1016/0277-5379(83)90419-4. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Daniels G. L., Shaw M. A., Lomas C. G., Leak M. R., Tippett P. The effect of In(Lu) on some high-frequency antigens. Transfusion. 1986 Mar-Apr;26(2):171–172. doi: 10.1046/j.1537-2995.1986.26286152909.x. [DOI] [PubMed] [Google Scholar]
- Doyle A., Jones T. J., Bidwell J. L., Bradley B. A. In vitro development of human monoclonal antibody-secreting plasmacytomas. Hum Immunol. 1985 Jul;13(3):199–209. doi: 10.1016/0198-8859(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Giles C. M. Antithetica+ relationship of anti-In-a with the Salis antibody. Vox Sang. 1975;29(1):73–76. doi: 10.1111/j.1423-0410.1975.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Harden E. A., Telen M. J., Hemler M. E., Strominger J. L., Palker T. J., Scearce R. M., Eisenbarth G. S. Differentiation of human T lymphocytes. I. Acquisition of a novel human cell surface protein (p80) during normal intrathymic T cell maturation. J Immunol. 1983 Sep;131(3):1195–1200. [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Judson P. A., Anstee D. J. Comparative effect of trypsin and chymotrypsin on blood group antigens. Med Lab Sci. 1977 Jan;34(1):1–6. [PubMed] [Google Scholar]
- Judson P. A., Spring F. A., Taylor M. A., Anstee D. J. Evidence for carbohydrate-deficient forms of the major sialoglycoproteins of human platelets, granulocytes and T lymphocytes in individuals with Tn syndrome. Immunology. 1983 Nov;50(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Letarte M., Iturbe S., Quackenbush E. J. A glycoprotein of molecular weight 85,000 on human cells of B-lineage: detection with a family of monoclonal antibodies. Mol Immunol. 1985 Feb;22(2):113–124. doi: 10.1016/s0161-5890(85)80005-5. [DOI] [PubMed] [Google Scholar]
- Mallinson G., Martin P. G., Anstee D. J., Tanner M. J., Merry A. H., Tills D., Sonneborn H. H. Identification and partial characterization of the human erythrocyte membrane component(s) that express the antigens of the LW blood-group system. Biochem J. 1986 Mar 15;234(3):649–652. doi: 10.1042/bj2340649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D. M., Kundu S. K., Suzuki A. The P blood group system: recent progress in immunochemistry and genetics. Semin Hematol. 1981 Jan;18(1):63–71. [PubMed] [Google Scholar]
- McKenzie J. L., Dalchau R., Fabre J. W. Biochemical characterisation and localization in brain of a human brain-leucocyte membrane glycoprotein recognised by a monoclonal antibody. J Neurochem. 1982 Nov;39(5):1461–1466. doi: 10.1111/j.1471-4159.1982.tb12592.x. [DOI] [PubMed] [Google Scholar]
- Merry A. H., Hodson C., Thomson E., Mallinson G., Anstee D. J. The use of monoclonal antibodies to quantify the levels of sialoglycoproteins alpha and delta and variant sialoglycoproteins in human erythrocyte membranes. Biochem J. 1986 Jan 1;233(1):93–98. doi: 10.1042/bj2330093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman P. C., Tippett P., Beal R. W. An Lu(a-b-) phenotype caused by an X-linked recessive gene. Vox Sang. 1986;51(1):49–52. doi: 10.1111/j.1423-0410.1986.tb00208.x. [DOI] [PubMed] [Google Scholar]
- Quackenbush E. J., Cruz T. F., Moscarello M. A., Letarte M. Identification of three antigens in human brain associated with similar antigens on human leukaemic cells. Biochem J. 1985 Jan 15;225(2):291–299. doi: 10.1042/bj2250291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush E. J., Gougos A., Baumal R., Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. J Immunol. 1986 Jan;136(1):118–124. [PubMed] [Google Scholar]
- Quackenbush E. J., Letarte M. Identification of several cell surface proteins of non-T, non-B acute lymphoblastic leukemia by using monoclonal antibodies. J Immunol. 1985 Feb;134(2):1276–1285. [PubMed] [Google Scholar]
- Spring F. A., Judson P. A., Daniels G. L., Parsons S. F., Mallinson G., Anstee D. J. A human cell-surface glycoprotein that carries Cromer-related blood group antigens on erythrocytes and is also expressed on leucocytes and platelets. Immunology. 1987 Oct;62(2):307–313. [PMC free article] [PubMed] [Google Scholar]
- Telen M. J., Eisenbarth G. S., Haynes B. F. Human erythrocyte antigens. Regulation of expression of a novel erythrocyte surface antigen by the inhibitor Lutheran In(Lu) gene. J Clin Invest. 1983 Jun;71(6):1878–1886. doi: 10.1172/JCI110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telen M. J., Palker T. J., Haynes B. F. Human erythrocyte antigens: II. The In(Lu) gene regulates expression of an antigen on an 80-kilodalton protein of human erythrocytes. Blood. 1984 Sep;64(3):599–606. [PubMed] [Google Scholar]
- Telen M. J., Shehata H., Haynes B. F. Human medullary thymocyte p80 antigen and In(Lu)-related p80 antigen reside on the same protein. Hum Immunol. 1986 Nov;17(3):311–324. doi: 10.1016/0198-8859(86)90283-1. [DOI] [PubMed] [Google Scholar]




