Abstract
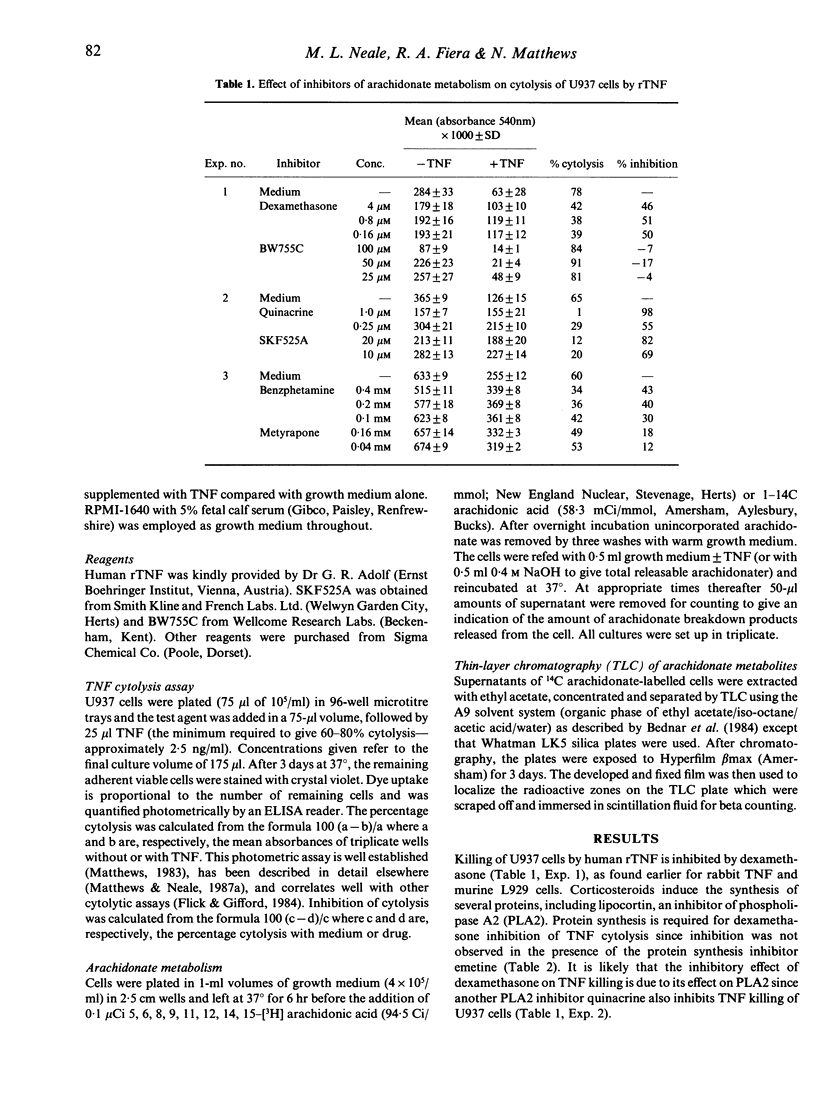
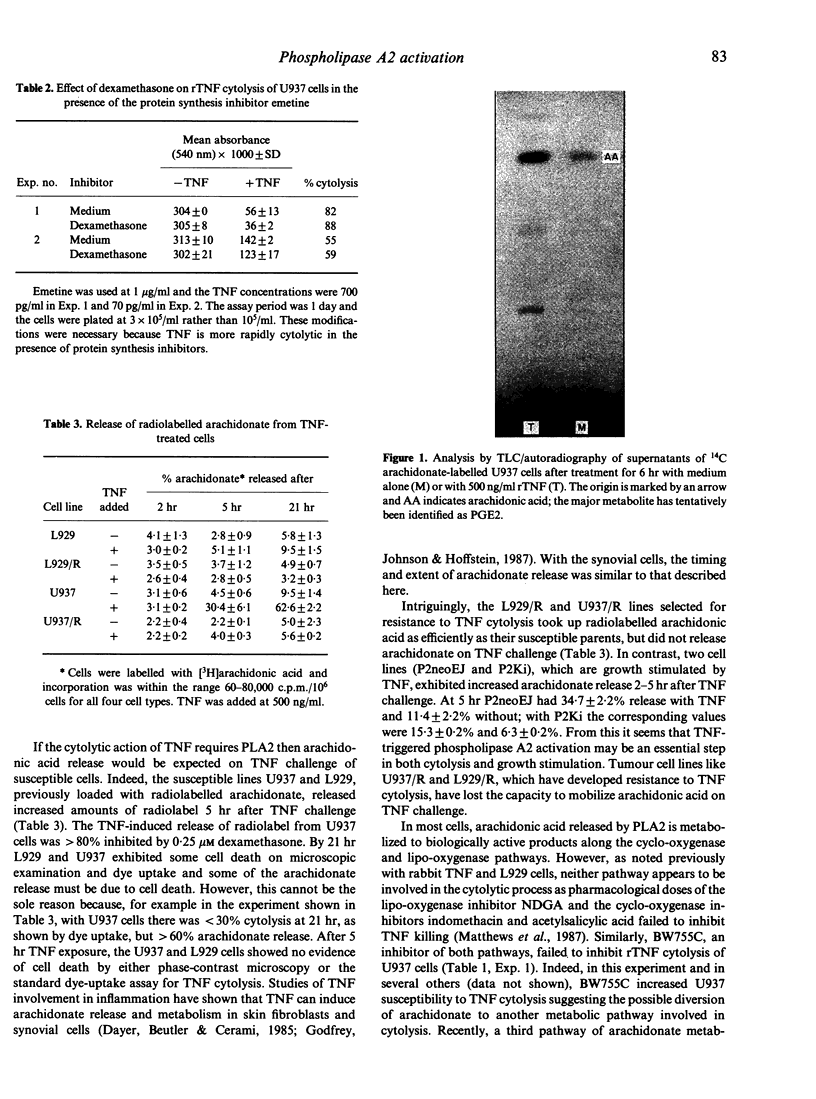
Earlier studies have indicated a possible role for arachidonate metabolism in the direct cytolysis of tumour cells by tumour necrosis factor (TNF) in vitro. In this study, the involvement of arachidonate metabolism has been investigated further with the following results: (i) Cytolysis of human U937 tumour cells by recombinant TNF was reduced by dexamethasone and quinacrine, agents which inhibit phospholipase A2. (ii) U937 and L929 cells, which are susceptible to TNF cytolysis, released arachidonic acid and its metabolites within 5 hr of TNF challenge, before cell death was apparent. In contrast, U937/R and L929/R, which are resistant to the cytolytic effects of TNF, did not release arachidonate products on TNF challenge. (iii) rTNF cytolysis of U937 cells was not reduced by inhibitors of the cyclo-oxygenase and lipo-oxygenase pathways of arachidonic acid metabolism. Cytolysis was reduced, however, by inhibitors of the arachidonate metabolic pathway involving cytochrome P450-dependent reductase, but only at reagent concentrations that also inhibited phospholipase A2 activity. Overall, these observations indicate a role for phospholipase A2 but not for arachidonic acid or its metabolites in the direct cytolysis of tumour cell lines by TNF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bednar M. M., Schwartzman M., Ibraham N. G., McGiff J. C., Mullane K. M. Conversion of arachidonic acid to two novel products by a cytochrome P450-dependent mixed-function oxidase in polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1984 Sep 17;123(2):581–588. doi: 10.1016/0006-291x(84)90269-9. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Chacos N., Werringloer J., Prough R. A., Estabrook R. W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Godfrey R. W., Johnson W. J., Hoffstein S. T. Recombinant tumor necrosis factor and interleukin-1 both stimulate human synovial cell arachidonic acid release and phospholipid metabolism. Biochem Biophys Res Commun. 1987 Jan 15;142(1):235–241. doi: 10.1016/0006-291x(87)90476-1. [DOI] [PubMed] [Google Scholar]
- Melera P. W., Hession C. A., Davide J. P., Scotto K. W., Biedler J. L., Meyérs M. B., Shanske S. Antifolate-resistant Chinese Hamster Cells. mRNA directed overproduction of multiple dihydrofolate reductases from a series of independently derived sublines containing amplified dihydrofolate reductase genes. J Biol Chem. 1982 Nov 10;257(21):12939–12949. [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumour necrosis factor. Polypeptide mediator network. 1987 Mar 26-Apr 1Nature. 326(6111):330–331. doi: 10.1038/326330a0. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Abelson H. T., Housman D. E., Howell N., Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984 Jun 14;309(5969):626–628. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Ferreri N. R., Carroll M. A., Songu-Mize E., McGiff J. C. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature. 1985 Apr 18;314(6012):620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]



