“Fair is foul and foul is fair” (so say the witches in the opening scene of Macbeth), and examples of this paradox abound in biology. Life-giving oxygen is also the source of cytotoxic oxidative stress; lethal gases like carbon monoxide are neurotransmitters that, among other things, facilitate reproduction (1). Another example is that of bilirubin, formerly dismissed as a waste product, whose image has been burnished by evolving evidence for a role in protecting cells from injury (2, 3). The latest development in this story, which focuses on the bilirubin-synthesizing enzyme biliverdin reductase (BVR), is the subject of an article by Barañano in this issue of PNAS (4).
The case has been made for a neuroprotective effect of bilirubin.
Bilirubin is best known as a yellow pigment contained in bile, which is released from the gall bladder into the duodenum to aid digestion. In Hippocratic medicine, yellow bile was one of four bodily fluids, or humors, that promoted health when in balance and illness when not. Humoral imbalances could also explain differences in personality: an excess of yellow bile made one choleric; black bile, melancholic; blood, sanguine; and phlegm, phlegmatic. It was not until the middle of the last century that the chemical structure of bilirubin was determined and the molecule was synthesized, by Hans Fischer, a German chemist. Fischer received the Nobel Prize in chemistry in 1930 for his work on the bilirubin precursor heme. He killed himself in 1945 when his Munich laboratories were destroyed in an Allied bombing raid on Nazi Germany.
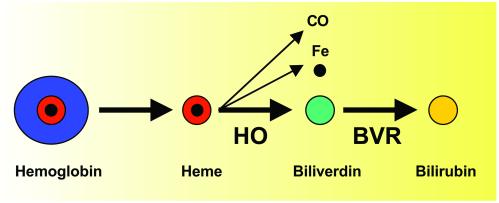
Bilirubin is produced as a by-product of the degradation of hemoglobin from senescent or hemolyzed red blood cells. The heme moiety of hemoglobin, in which iron is coordinated to each of four pyrrole rings of protoporphyrin, enters the blood and is transported to the liver. There, and in most other tissues, heme is metabolized by the microsomal enzyme heme oxygenase 1 (HO1) in the presence of nicotinamide-adenine dinucleotide phosphate (NADPH) and oxygen, to produce biliverdin, carbon monoxide, and iron. In other tissues, including the brain, another isoform of heme oxygenase, HO2, is involved. Following its synthesis by HO1 or HO2, biliverdin is converted by the phosphoprotein BVR (5), in the presence of NADPH, to bilirubin (Fig. 1). The crystal structures of the principal fetal (biliverdin IXβ reductase, or BVRB) and adult (biliverdin IXα reductase, or BVRA) forms of BVR have been reported recently (6, 7).
Fig. 1.
Synthesis of bilirubin from heme. HO, heme oxygenase; BVR, biliverdin reductase; CO, carbon monoxide.
The clinician confronts bilirubin in several settings. It is responsible for yellow discoloration of the skin in physiological jaundice of the newborn (Fig. 2) and in erythroblastosis fetalis, which usually results from Rh blood group incompatibility between an Rh-negative mother and an Rh-positive neonate. In this disorder, bilirubin accumulates as a result of antibody-induced hemolysis, which releases hemoglobin that is sequentially converted to heme, biliverdin, and bilirubin. Treatment involves phototherapy and exchange transfusion to prevent kernicterus, in which high concentrations of bilirubin gain access to the basal ganglia and other brain structures, resulting in lethargy, rigidity, seizures, death, and, in long-term survivors, choreoathetosis, hearing loss, and Parinaud's syndrome (paralysis of upward gaze; ref. 8). Jaundice is also seen in hepatocellular disorders and extrahepatic biliary obstruction, which lead to increased bilirubin levels in the blood and its subsequent deposition in elastin-rich tissues, such as skin and sclera. Finally, because bilirubin is derived from hemoglobin, it is seen at sites of recent bleeding, such as hematomas under the skin. A special case is xanthochromia, or yellow coloration, of the cerebrospinal fluid, which every medical student learns to recognize as a sign of subarachnoid hemorrhage.
Fig. 2.

Physiological jaundice of the newborn (photo courtesy of Maeve Greenberg).
The example of kernicterus, in particular, suggests that bilirubin is cytotoxic. Several studies have shown such toxicity (9), which typically occurs at micromolar concentrations of bilirubin. Bilirubin causes death of cultured neurons (10) and cerebral microvascular endothelial cells (11) in vitro, and this has features of apoptotic cell death, including DNA fragmentation, release of cytochrome c, activation of caspase-3, and cleavage of poly(ADP)ribose polymerase. N-methyl-d-aspartate receptor antagonists can protect cultured neurons from bilirubin toxicity (12), implicating this class of glutamate receptors in pathogenesis. Bilirubin may also be an indirect cause of neuronal death after subarachnoid hemorrhage. Bilirubin oxidation products, derived from blood that enters the subarachnoid space when aneurysms rupture, cause cerebral vasospasm (13), and this leads, in turn, to morbidity and mortality from delayed cerebral ischemia or infarction.
Accumulating evidence points to a protective role of bilirubin as well. Stocker et al. (14) showed that bilirubin is an antioxidant that can scavenge peroxyl radicals, and bilirubin has been reported to protect against a variety of pathological processes, including complement-mediated anaphylaxis (15), myocardial ischemia (16), pulmonary fibrosis (17), and cyclosporin nephrotoxicity (18).
In a series of papers from the Snyder laboratory, the case has been made for a neuroprotective effect of bilirubin. Doré et al. (19) showed that hydrogen peroxide toxicity was increased in hippocampal neuron cultures from HO2-knockout mice, and that addition of free bilirubin (25–50 nM), or bilirubin conjugated to albumin (10–250 nM), improved survival. They later reported that after focal cerebral ischemia induced by occlusion of the middle cerebral artery followed by reperfusion, or intracerebral injection of the excitotoxic amino acid N-methyl-d-aspartate, injury was more extensive in HO2-knockout (but not HO1-knockout) than in wild-type mice, which is consistent with a role for the products of HO2 in protection from ischemic injury (20). The failure of HO1-knockouts to show increased injury may be related to the nonneuronal location of HO1. In a follow-up study, the form of cell death against which HO2 protects was investigated (21). Death was induced in cultured cerebellar granule neurons by withdrawing serum and reducing the extracellular concentration of potassium. In both wild-type and HO2-knockout mice, cell death was predominantly apoptotic, as judged by morphological features including nuclear fragmentation and apoptotic bodies. However, cell death was approximately two-fold greater in cultures from HO2 knockouts, and this difference was accounted for almost completely by apoptosis. Focal cerebral ischemia in HO2-knockout mice or wild-type mice treated with the HO2 inhibitor tin protoporphyrin IX also increased the number of cells with apoptotic morphology in the ischemic penumbra. In addition to these results implicating HO2 in protection from cerebral ischemia, an intriguing connection to Alzheimer's disease has been made. Takahashi et al. (22) used yeast two-hybrid analysis and coimmunoprecipitation studies to identify proteins that interact with HO2. They found that one such protein was amyloid precursor protein (APP), which is the source of β-amyloid in Alzheimer's disease, and which is mutated in some familial forms of the disorder. Interaction with wild-type APP inhibited the activity of HO2, but the Swedish, Dutch, and London APP mutations had about twice the inhibitory effect of wild-type APP. Moreover, cortical neuron cultures from mice expressing the Swedish mutation showed defects in bilirubin production and enhanced toxicity from hydrogen peroxide. The authors concluded that HO may help to regulate oxidative injury in Alzheimer's disease, which is one way that heme deficiency could impact the disease, as proposed recently (23). These and related studies have been reviewed (24).
In this issue of PNAS, Barañano et al. (4) ask, “As biliverdin is water-soluble and readily excreted, why should mammals have evolved the energetically expensive, potentially toxic, and apparently unnecessary capacity to reduce biliverdin (to bilirubin)?” The simple answer, from their own prior work, is that bilirubin is neuroprotective, but the low (nanomolar) concentrations of bilirubin present in cells, and the high concentrations of oxidants against which they protect, must be reconciled. To explain this discrepancy, Barañano et al. hypothesize that a mechanism must exist to amplify the antioxidant effect of bilirubin.
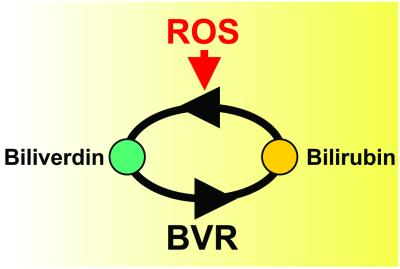
The mechanism they propose involves redox cycling (Fig. 3). Biliverdin is reduced to bilirubin through the action of BVR: bilirubin interacts with reactive oxygen species (ROS), which neutralizes their toxicity and oxidizes bilirubin, thereby regenerating biliverdin. As this cycle is repeated, the antioxidant effect of bilirubin is multiplied. This scheme gives rise to at least two testable predictions. First, ROS should promote the synthesis of biliverdin. Second, depletion of BVR should increase levels of ROS and their toxic effects. To test the effect of ROS on biliverdin synthesis, Barañano et al. treated HeLa cells with a compound that generates ROS, and found that biliverdin was produced. To determine the effect of BVR depletion on ROS activity, they used RNA interference and succeeded in reducing BVR activity in HeLa cells to 5–10% of normal levels. Oxidative activity, measured by a fluorescence assay, increased by ≈200%. Similar results were obtained with cultured cortical neurons. BVR-depleted cells were also more susceptible to caspase-dependent death from hyperoxia and to hydrogen peroxide toxicity, which is consistent with an inability to recycle bilirubin and amplify its antioxidant effect. Finally, the antioxidant activity of BVR was quantitatively comparable to that of glutathione, leading the authors to suggest that BVR and glutathione may be the principal endogenous antioxidants associated with the membrane and cytoplasmic compartments, respectively.
Fig. 3.
Amplification of the neuroprotective effect of bilirubin by redox cycling. Biliverdin is reduced to bilirubin by biliverdin reductase (BVR) and is regenerated when the detoxification of reactive oxygen species (ROS) oxidizes bilirubin back to biliverdin. In this manner, low concentrations of bilirubin can be recycled to neutralize large amounts of ROS.
A common feature of endogenous neuroprotective systems is that many of them are transcriptionally induced by the injury states against which they protect. In cerebral ischemia, for example, increased expression in the ischemic penumbra, a region that can be salvaged by endogenous or exogenous neuroprotectants, is observed for such diverse protective proteins as heat-shock proteins, growth factors, hypoxia-inducible factor-1 and its targets, and anti-apoptotic Bcl-2-family gene products (25, 26), as well as the recently identified oxygen-binding protein, neuroglobin (27). It is of interest, therefore, that BVR expression is also increased in the penumbra after focal cerebral ischemia (28), which may be further evidence of its neuroprotective role.
An additional function for BVR has been identified. The protein contains a leucine zipper DNA-binding motif and, in homodimeric form, binds to a region of the HO1 promoter that contains two AP-1 sites (29). Therefore, BVR may protect cells not only by catalyzing the formation of bilirubin, but also by transcriptional activation of HO1, which promotes the efflux of potentially toxic iron from cells exposed to oxidative stress (24).
In a 1990 review titled “Is bilirubin good for you?”, McDonagh (30) summarized the evidence for a protective role of bilirubin, concluding that “the biochemical path from red (heme) to green (biliverdin) to yellow (bilirubin) may defend as well as degrade.” Barañano et al. (4) have provided important additional evidence for this view by demonstrating a mechanism that makes it quantitatively plausible, thereby spotlighting a potential therapeutic target in stroke and other disorders associated with oxidative injury.
See companion article on page 16093.
References
- 1.Burnett A. L., Johns, D. G., Kriegsfeld, L. J., Klein, S. L., Calvin, D. C., Demas, G. E., Schramm, L. P., Tonegawa, S., Nelson, R. J., Snyder, S. H. & Poss, K. D. (1998) Nat. Med. 4, 84-87. [DOI] [PubMed] [Google Scholar]
- 2.Tomaro M. L. & Batlle, A. M. (2002) Int. J. Biochem. Cell Biol. 34, 216-220. [DOI] [PubMed] [Google Scholar]
- 3.Marilena G. (1997) Biochem. Mol. Med. 61, 136-142. [DOI] [PubMed] [Google Scholar]
- 4.Barañano D. E., Rao, M., Ferris, C. D. & Snyder, S. H. (2002) Proc. Natl. Acad. Sci. USA 99, 16093-16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salim M., Brown-Kipphut, B. A. & Maines, M. D. (2001) J. Biol. Chem. 276, 10929-10934. [DOI] [PubMed] [Google Scholar]
- 6.Pereira P. J., Macedo-Ribeiro, S., Parraga, A., Perez-Luque, R., Cunningham, O., Darcy, K., Mantle, T. J. & Coll, M. (2001) Nat. Struct. Biol. 8, 215-220. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi A., Park, S. Y., Miyatake, H., Sun, D., Sato, M., Yoshida, T. & Shiro, Y. (2001) Nat. Struct. Biol. 8, 221-225. [DOI] [PubMed] [Google Scholar]
- 8.Connolly A. M. & Volpe, J. J. (1990) Clin. Perinatol. 17, 371-379. [PubMed] [Google Scholar]
- 9.Hansen T. W. (2001) J. Perinatol. 21, Suppl. 1, S48-S62. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues C. M., Sola, S. & Brites, D. (2002) Hepatology 35, 1186-1195. [DOI] [PubMed] [Google Scholar]
- 11.Akin E., Clower, B., Tibbs, R., Tang, J. & Zhang, J. (2002) Brain Res. 931, 168-175. [DOI] [PubMed] [Google Scholar]
- 12.Grojean S., Koziel, V., Vert, P. & Daval, J. L. (2000) Exp. Neurol. 166, 334-341. [DOI] [PubMed] [Google Scholar]
- 13.Clark J. F., Reilly, M. & Sharp, F. R. (2002) J. Cereb. Blood Flow Metab. 22, 472-478. [DOI] [PubMed] [Google Scholar]
- 14.Stocker R., Yamamoto, Y., McDonagh, A. F., Glazer, A. N. & Ames, B. N. (1987) Science 235, 1043-1046. [DOI] [PubMed] [Google Scholar]
- 15.Nakagami T., Toyomura, K., Kinoshita, T. & Morisawa, S. (1993) Biochim. Biophys. Acta 1158, 189-193. [DOI] [PubMed] [Google Scholar]
- 16.Clark J. E., Foresti, R., Sarathchandra, P., Kaur, H., Green, C. J. & Motterlini, R. (2000) Am. J. Physiol. 278, H643-H651. [DOI] [PubMed] [Google Scholar]
- 17.Wang H. D., Yamaya, M., Okinaga, S., Jia, Y. X., Kamanaka, M., Takahashi, H., Guo, L. Y., Ohrui, T. & Sasaki, H. (2002) Am. J. Respir. Crit. Care Med. 165, 406-411. [DOI] [PubMed] [Google Scholar]
- 18.Polte T., Hemmerle, A., Berndt, G., Grosser, N., Abate, A. & Schroder, H. (2002) Free Radical Biol. Med. 32, 56-63. [DOI] [PubMed] [Google Scholar]
- 19.Dore S., Takahashi, M., Ferris, C. D., Zakhary, R., Hester, L. D., Guastella, D. & Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 2445-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dore S., Sampei, K., Goto, S., Alkayed, N. J., Guastella, D., Blackshaw, S., Gallagher, M., Traystman, R. J., Hurn, P. D., Koehler, R. C. & Snyder, S. H. (1999) Mol. Med. 5, 656-663. [PMC free article] [PubMed] [Google Scholar]
- 21.Dore S., Goto, S., Sampei, K., Blackshaw, S., Hester, L. D., Ingi, T., Sawa, A., Traystman, R. J., Koehler, R. C. & Snyder, S. H. (2000) Neuroscience 99, 587-592. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M., Dore, S., Ferris, C. D., Tomita, T., Sawa, A., Wolosker, H., Borchelt, D. R., Iwatsubo, T., Kim, S. H., Thinakaran, G., et al. (2000) Neuron 28, 461-473. [DOI] [PubMed] [Google Scholar]
- 23.Atamna H., Killilea, D. W., Killilea, A. N. & Ames, B. N. (2002) Proc. Natl. Acad. Sci. USA 99, 14807-14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barañano D. E. & Snyder, S. H. (2001) Proc. Natl. Acad. Sci. USA 98, 10996-11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp F. R., Lu, A., Tang, Y. & Millhorn, D. E. (2000) J. Cereb. Blood Flow Metab. 20, 1011-1032. [DOI] [PubMed] [Google Scholar]
- 26.Graham S. H. & Chen, J. (2001) J. Cereb. Blood Flow Metab. 21, 99-109. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y., Jin, K., Mao, X. O., Zhu, Y. & Greenberg, D. A. (2001) Proc. Natl. Acad. Sci. USA 98, 15306-15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panahian N., Huang, T. & Maines, M. D. (1999) Brain Res. 850, 1-13. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad Z., Salim, M. & Maines, M. D. (2002) J. Biol. Chem. 277, 9226-9232. [DOI] [PubMed] [Google Scholar]
- 30.McDonagh A. F. (1990) Clin. Perinatol. 17, 359-369. [PubMed] [Google Scholar]