Ever since Paul Ehrlich introduced the term “magic bullet,” the exquisite capacity for specificity afforded by the immune system has always underpinned its appeal as a therapeutic weapon against cancer. The first clinical validation of this principle came in the form of mAb administration, which, after a decade of skepticism, produced therapeutic successes in breast cancer and B cell lymphomas. T cell-based immunotherapy offers an even broader therapeutic potential, owing to the ability of T cells to recognize peptides derived from proteins in any cellular compartment. These peptides are produced when proteins are processed by specialized machinery; they then combine with MHC molecules, which transport them to the cell surface where the peptide–MHC complexes can be recognized by T cells. Although hundreds of experiments in rodent tumor models support the notion that tumor-specific T cells can be activated to inhibit tumor growth, direct evidence for therapeutic capacity in human cancer has been marginal. Now, two clinical studies using adoptive transfer of melanoma-specific T cells provide clear evidence for the ability of T cells to mediate antitumor activity and provide important general principles for T cell immunotherapy (1, 2).
The most direct approach for analyzing the activity of tumor-reactive T cells is to grow T cell clones ex vivo with defined specificity and adoptively transfer them back into the tumor-bearing host. It is typically much more difficult to grow tumor-reactive T cells from cancer patients than to grow virus-reactive T cells. This has been thought to be because the immune system views tumors more as “self” than foreign; thus, T cells become tolerant to tumor antigens. Nonetheless, tumor tolerance is relative and not absolute, leaving open a potential window of immunotherapeutic opportunity (3). Melanoma has been the most popular target for T cell-based immunotherapy in part because it is much easier to grow tumor-reactive T cells from melanoma patients than any other type of human cancer. These T cells have been be used to define specific antigens recognized by T cells. A surprising finding to fall out of these antigen discovery efforts is that the melanoma antigens most commonly recognized are not tumor specific, but rather are tissue-specific melanocyte antigens such as tyrosinase, MART-1/MelanA, and gp100 (4). Thus, at least in melanoma, tumor immunity is in part synonymous with tissue-specific autoimmunity. This autoreactivity is not an absolute barrier to tumor immunotherapy because many of the common cancers such as prostate cancer, breast cancer, pancreatic cancer, and melanoma arise from tissues dispensable to life.
Yee et al. demonstrated that adoptively transferred T cell clones could persist in vivo and further could traffic into tumors.
The ability to grow melanocyte-specific T cells from melanoma patients does not mean that in vivo tolerance has been broken as a consequence of tumorigenesis. Many of the T cell clones grown from these patients recognize peptides that bind poorly to their presenting MHC allele or possess T cell receptors with relatively low affinity for their cognate peptide–MHC complex. These T cells thus recognize melanoma/melanocyte antigens weakly compared with typical virus-specific T cells and presumably fail to become activated in vivo, thereby “ignoring” melanocytes and melanoma cells. Evidence from animal models suggests that high-affinity, tumor-reactive T cells are more actively tolerized than weaker low-affinity T cells by mechanisms involving deletion, anergy induction, or suppression (5).
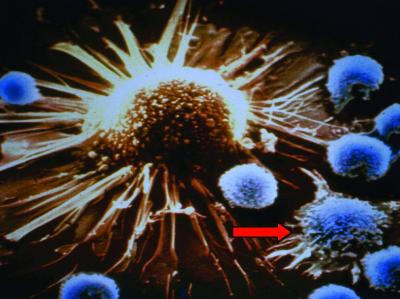
Given the emerging view that individuals with cancer contain tumor-reactive T cells that are naturally tolerant of their cancer, a central question in T cell immunotherapy is whether they can be activated and expanded to induce clinically useful antitumor responses. Even low-affinity T cells can potentially discharge their effector function if properly activated. For example, CD8 T cells that recognize peptides presented by MHC class I molecules require >100 peptide–MHC complexes plus multiple costimulatory signals to become primed; however, once activated (to so-called killer T cells or cytotoxic T lymphocytes) they can kill target cells expressing as few as one peptide–MHC complex in the absence of any additional accessory signals (6, 7) (Fig. 1).
Fig 1.
T cell recognition of cancer. In this scanning electron micrograph, a tumor-specific CD8+ killer T cell is shown in the act of recognizing a tumor cell expressing the cognate tumor antigen (arrow). Multiple other T cells not specific for any tumor antigens ignore the tumor cell. Tumor antigen recognition first involves generation of a tight junction with the tumor cell after which perforin is released to create pores in the tumor target. Granzymes released by the killer T cell can enter the cytoplasm of the tumor via these pores and activate effector caspases to induce apoptosis. (Magnification: ×5,000.)
To address the in vivo activity of melanoma-specific T cells, Yee et al. (1) grew T cell clones from patients with advanced melanoma specific for two well-defined melanoma/melanocyte antigens, MART-1/Melan A or gp100. They initially primed T cells in vitro by using peptide-loaded dendritic cells, the most powerful stimulator of immune responses. Many of the T cell clones grown under these conditions fail to kill tumor cells even when activated, reflecting the generally low affinities of T cells remaining after tolerance has operated on the available repertoire. However, a subset of CD8 T cell clones were indeed capable of killing autologous tumor cells, confirming the existence of tumor-reactive T cells in vivo that could be activated and expanded in vitro. T cell clones capable of recognizing tumor were rapidly expanded in culture (using an anti-CD3 antibody that stimulates through the T cell receptor signaling pathway) and roughly 6 billion cells with pure clonal specificity were transferred back to patients every 2–3 weeks. The first infusion was given without any systemic IL-2, and the subsequent infusions were given with relatively low doses of IL-2 that should activate the high affinity IL-2 receptor (α+β+γ chains) expressed on activated T cells. IL-2 is a T cell growth factor produced by CD4 helper T cells.
A number of important insights can be gleaned from the analysis of the 10 treated patients in the Yee study. First and foremost, they demonstrated that adoptively transferred T cell clones could persist in vivo and further could traffic into tumors. T cell clones were tracked in vivo by staining cells with peptide–MHC tetramers that exhibit specific and stable binding to cognate T cells, thereby allowing flow cytometric analysis (8). The persistence of adoptively transferred T cell clones in the peripheral blood was surprisingly consistent among all of the patients. Thus, the half-life for adoptively transferred T cells in the absence of systemically delivered IL-2 (first infusion) was roughly 1 week, whereas the half-life was significantly extended to ≈2.5 weeks in the presence of systemically administered IL-2 (infusions 2, 3, and 4). This finding confirms earlier conclusions from adoptive transfer studies with cytomegalovirus-specific T cells that persistence of antigen-specific CD8 cells requires “help” either through antigen-specific CD4 cells or via systemic administration of helper-derived cytokines, such as IL-2 (9). The findings further support the value of “low-dose” IL-2 in the setting of antigen-specific immunotherapy. This issue has been somewhat controversial because a number of recent studies have suggested that IL-2 ultimately might inhibit the generation of long-term memory T cells. A caveat in the analysis of peripheral blood alone is that the systemically administered IL-2 might simply alter the relative distribution of antigen-specific CD8 cells between lymph nodes, peripheral tissues, and peripheral blood. Indeed, it is now appreciated that activated T cells are divisible into different subsets characterized by predominant residence in secondary lymphoid tissues (central memory cells) versus peripheral tissues and peripheral blood (effector memory cells) (10).
Importantly, analysis of tumor biopsies after T cell administration revealed a dramatic concentration of adoptively transferred antigen-specific T cells within sites of metastatic tumor. In one example, 37% of the tumor-infiltrating CD8 cells stained positive with the appropriate MHC tetramer at a time when <1% of the CD8 cells in peripheral blood stained positive. The clinical course of treated patients suggested a modest clinical benefit of T cell transfer with disease stabilization in roughly half of the cases. However, there were no formal partial clinical responses (defined as >50% reduction in cross-sectional area of all measurable metastases) or complete responses (defined as regression of all measurable metastatic disease). None of the patients demonstrated true vitiligo (autoimmune depigmentation) suggestive of widespread melanocyte destruction, although one patient did develop a targetoid erythema around nontumorous pigmented lesions, which on biopsy was infiltrated with lymphocytes. Does the absence of significant tumor regressions in the setting of successful traffic of adoptively transferred T cells into tumor masses suggest that they are incapable of antitumor activity upon arrival at metastatic sites? Evaluation of antigen expression in biopsies of tumor sites preadoptive and postadoptive T cell transfer in fact suggests that this is not the case. Thus, immunohistochemical staining for three different melanosomal antigens (tyrosinase, MART1/MelanA, and gp100) demonstrated selective loss of the melanosomal antigen targeted by the adoptively transferred T cells in three of five analyzed patients. This high frequency of treatment-induced selection for antigen loss variants strongly supports the activity of T cells against tumor cells expressing the target antigen. However, as with other therapeutic approaches, the genetic instability of cancer provides it with the frustrating capacity to circumvent attack by therapies that are too narrowly focused and targeted against molecules or pathways dispensable to the growth and survival of the tumor.
In parallel studies, Rosenberg and colleagues (2) have also evaluated the efficacy of adoptively transferred melanoma-specific T cells. In a “back to the future” move, they have returned to using tumor-infiltrating lymphocytes (TIL) for adoptive therapy, abandoning the use of individual CD8 clones. The updated version of TIL therapy bears a number of important differences from the original TIL therapy of the 1990s. First, a number of TIL cultures (grown by incubating dissociated tumor resection specimens in IL-2) were initiated for each patient and only those that generated documented tumor-specific reactivity in vitro were expanded [using the anti-CD3 protocol of Riddell and Greenberg (14)]. Thus, the infused T cells were more highly enriched for tumor-specific T cells than the original TIL protocols. Nonetheless, in contrast to the Yee trial, the infused cells of the Dudley trial were polyclonal and indeed quite variable from patient to patient. Although most of the cultures contain documented MART1/MelanA reactivity or gp100 reactivity, the composition of infused T cells varied from 85% CD4+ T cells to 96% CD8+ T cells among different patients. Another major feature of this recent TIL protocol was the use of lymphoablative preconditioning of patients with cytoxan/fludarabine chemotherapy combinations. The rationale for this maneuver was that lymphoablation before reinfusion of lymphocytes would result in endogenous production of various growth factors (such as IL-7 and IL-15) that would enhance the expansion of adoptively transferred cells to “fill the space” (11, 12). With this protocol, Dudley et al. report impressive clinical responses; 6 of 13 patients achieved true partial responses and an additional 4 of 13 patients displayed mixed responses (defined as some metastases shrinking while others grew). The overall enhanced potency of this approach is further suggested by the high frequency of true vitiligo induction in this cohort (4 of 13 patients). Although not perfect, there is a moderate correlation between vitiligo induction and tumor response in many forms of melanoma immunotherapy, emphasizing the interrelationship between autoimmunity and tumor immunity (13).
Two of the most interesting patients in the Dudley study developed a lymphocytosis post-TIL infusion that was characterized by predominant representation of a single T cell clone from the TIL culture (60% of the total peripheral blood CD8 T cells in one patient and 75% of the CD8 T cells in another patient). Surprisingly, these dominant T cell clones appeared to persist for many months even after discontinuation of systemic IL-2. Other than leukemogenic transformation, the long-term stable persistence of a single T cell clone representing >50% of the total T cell pool is highly unusual and represents a dramatic break in T cell repertoire homeostasis. Although establishment of such drastic in vivo clonal dominance indeed resulted in a strong antitumor effect, it may also be somewhat immunosuppressive for the remaining repertoire; indeed, one of the two patients developed an AIDS-like immunodeficiency characterized by Epstein–Barr virus lymphomagenesis and multiple debilitating opportunistic infections.
What can we learn from these studies specifically regarding adoptive T cell therapy and cancer immunotherapy in general? Although it is difficult to compare the Yee and Dudley studies directly, owing to the large number of major differences in the adoptive transfer protocols, a number of unifying conclusions can at least be tentatively drawn. Clearly, these studies indicate that appropriately activated T cells are indeed capable of trafficking into even large metastatic tumor deposits and eliminating tumor cells expressing the target antigen. Second, unless the T cells are specific for a target critical to the tumors growth and survival, a monovalent specificity to the immunotherapy is likely to be insufficient in patients with large tumor burden. This conclusion is strongly supported by the high rate of development of antigen loss variants subsequent to adoptive therapy with a single CD8 T cell clone. It is likely that at least part of the enhanced clinical success of the Dudley study relates to the mixed specificities of the infused TIL cultures. In particular, the majority of infused TIL contain a significant number of CD4 T cells, which were completely absent from the adoptively transferred CD8 clonal populations in the Yee study. There is much evidence that a combination of CD4 and CD8 responses is critical to generating optimal levels of the sustained memory and antitumor immunity (14–16). Thus, future immunotherapy strategies need to focus on the generation of combined CD4 and CD8 T cell activation. The role of lymphoablation used in the Dudley study certainly bears further evaluation. Lymphoablation is not the sole factor as an earlier study of adoptively transferring gp100-specific T cell clones into similarly lymphoablated patients failed to generate any objective clinical responses (17). Nonetheless, it is likely that the lymphoablation procedure was responsible for the lymphocytosis/clonal dominance phenomenon observed in the two patients on the recent protocol. A number of preclinical studies evaluating immunization after autologous bone marrow transplantation demonstrated an increased burst size of antigen-specific T cells when immunization was performed in posttransplant lymphopenic hosts in the process of immunologic recovery (18). Ultimately, understanding the specific signals involved in homeostatic T cell proliferation (the T cell expansion that occurs in the context of depleted T cell compartments) will likely yield important information that can be used to potentially enhance the expansion and activation of antigen-specific T cells in immunotherapy. Whether the homeostatic expansion process can break antigen-specific tolerance remains to be determined. Clinical studies using IL-7 are in the process of being designed. IL-7 is likely an important proliferative factor involved in both homeostatic T cell proliferation as well as the late-stage chronic proliferation of the memory T cell pool. Finally, it is important to remember that when it comes to the efficacy of tumor-reactive T cells quality is just as important as quantity. Flooding the system with relatively ineffective, low-affinity T cells is unlikely to generate a sustained antitumor response. Factors such as homing capacity, activation state, and in particular T cell affinity must ultimately be optimized for immunotherapy to be successful. As our ability to quantitatively measure these parameters of T cell function continuously improves, so, too, will the opportunities for improved immunotherapy of cancer.
See companion article on page 16168.
References
- 1.Yee C., Thompson, J. A., Byrd, D., Riddell, S. R., Roche, P., Celis, E. & Greenberg, P. D. (2002) Proc. Natl. Acad. Sci. USA 99, 16168-16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley M. E., Wunderlich, J. R., Robbins, P. F., Yang, J. C., Hwu, P., Schwartzentruber, D. J., Topalian, S. L., Sherry, R., Restifo, N. P., Hubicki, A. M., et al. (2002) Science 298, 850-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll D. M. (2002) Nat. Rev. Immunol. 2, 227-238. [DOI] [PubMed] [Google Scholar]
- 4.Boon T., Cerottini, J. C., Van den Eynde, B., van der Bruggen, P. & Van Pel, A. (1994) Annu. Rev. Immunol. 12, 337-365. [DOI] [PubMed] [Google Scholar]
- 5.Levitsky H. I. (2000) Cancer J. 3, S281-S290. [PubMed] [Google Scholar]
- 6.Viola A. & Lanzavecchia, A. (1996) Science 273, 104-106. [DOI] [PubMed] [Google Scholar]
- 7.Sykulev Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. (1996) Immunity. 4, 565-571. [DOI] [PubMed] [Google Scholar]
- 8.Altman J. D., Moss, P. A., Goulder, P. J., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274, 94-96. [DOI] [PubMed] [Google Scholar]
- 9.Riddell S. R., Watanabe, K. S., Goodrich, J. M., Li, C. R., Agha, M. E. & Greenberg, P. D. (1992) Science 257, 238-241. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. (1999) Nature 401, 708-712. [DOI] [PubMed] [Google Scholar]
- 11.Tan J. T., Ernst, B., Kieper, W. C., LeRoy, E., Sprent, J. & Surh, C. D. (2002) J. Exp. Med. 195, 1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan J. T., Dudl, E., LeRoy, E., Murray, R., Sprent, J., Weinberg, K. I. & Surh, C. D. (2001) Proc. Natl. Acad. Sci. USA 98, 8732-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg S. A. & White, D. E. (1996) J. Immunother. Emphasis Tumor Immunol. 19, 81-84. [PubMed] [Google Scholar]
- 14.Riddell S. R. & Greenberg, P. D. (1995) Annu. Rev. Immunol. 13, 545-586. [DOI] [PubMed] [Google Scholar]
- 15.Hung K., Hayashi, R., Lafond-Walker, A., Lowenstein, C., Pardoll, D. & Levitsky, H. (1998) J. Exp. Med. 188, 2357-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardoll D. M. & Topalian, S. L. (1998) Curr. Opin. Immunol. 10, 588-594. [DOI] [PubMed] [Google Scholar]
- 17.Dudley M. E., Wunderlich, J. R., Yang, J. C., Hwu, P., Schwartzentruber, D. J., Topalian, S. L., Sherry, R. M., Marincola, F. M., Leitman, S. F., Seipp, C. A., et al. (2002) J. Immunother. 25, 243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borrello I., Sotomayor, E. M., Rattis, F. M., Cooke, S. K., Gu, L. & Levitsky, H. I. (2000) Blood 95, 3011-3019. [PubMed] [Google Scholar]