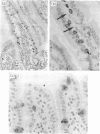
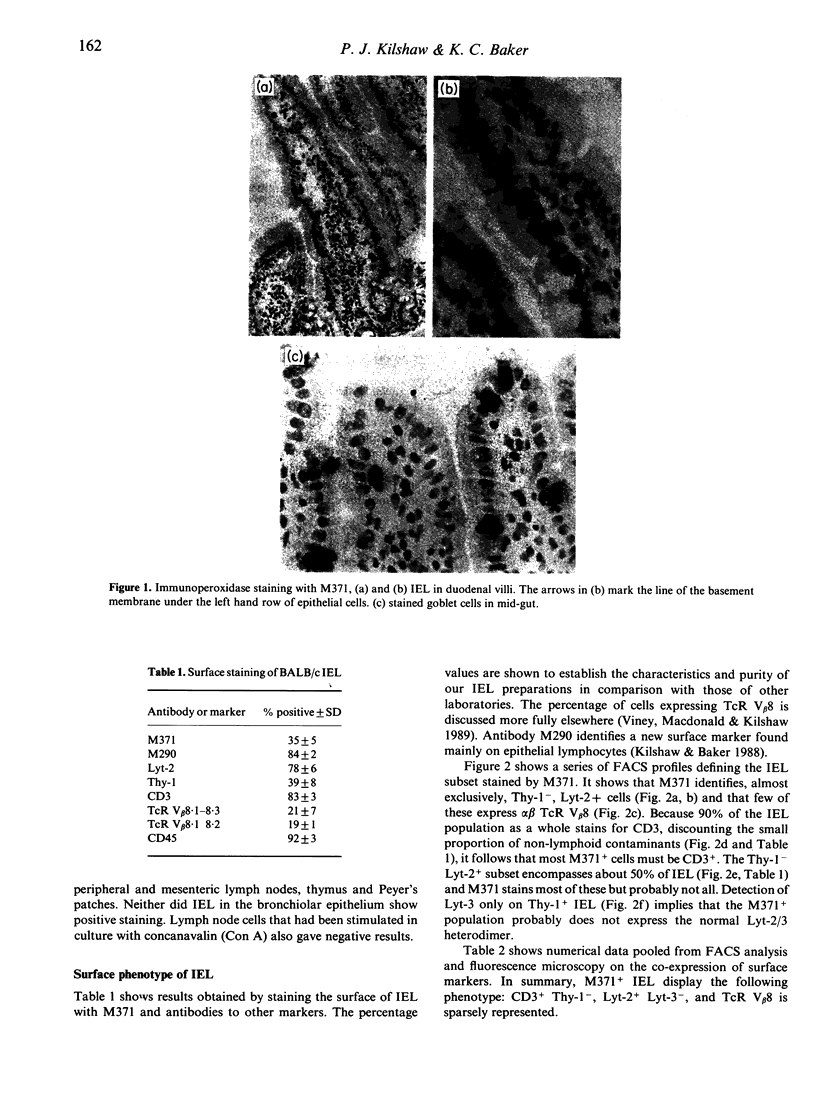
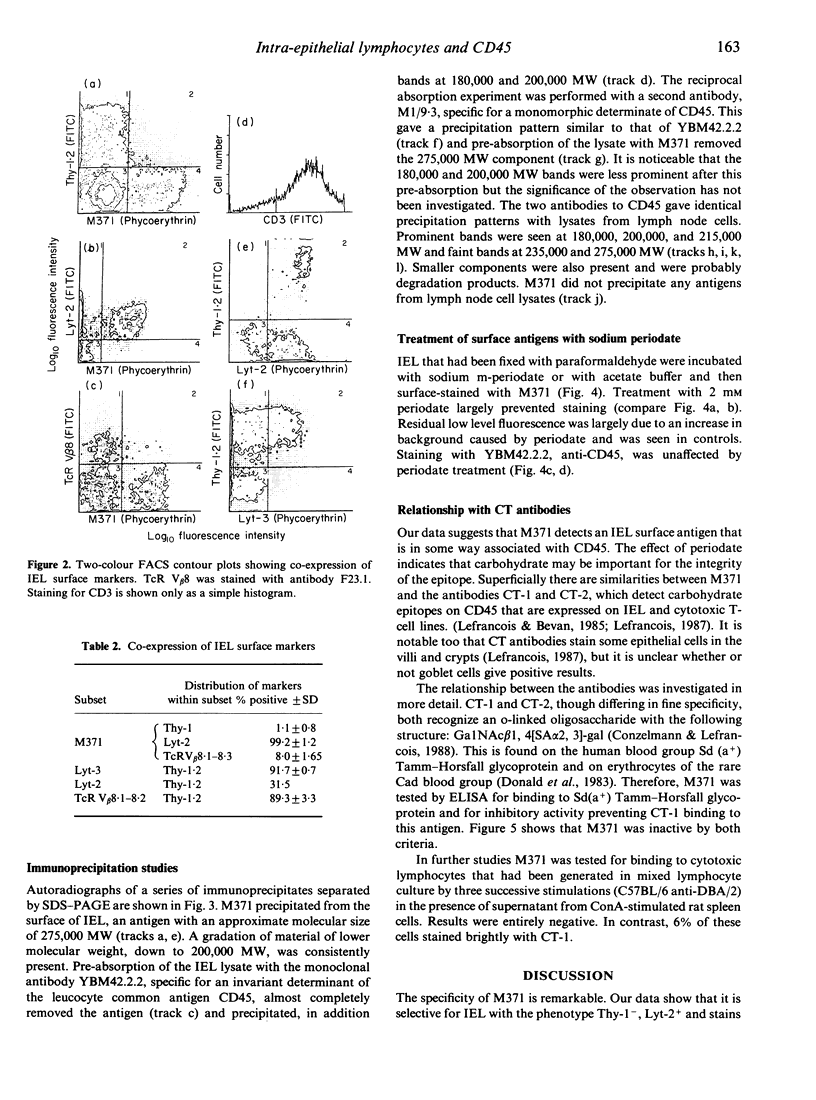
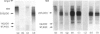Abstract
Rat monoclonal antibodies were prepared against intra-epithelial lymphocytes (IEL) isolated from the gut of Balb/c mice and screened for selective reactivity with mucosal lymphocytes. One antibody, M371, identified a new surface antigen on 30-40% of IEL. It bound to very few, if any, lymphocytes within the lamina propria and to none in other lymphoid tissues; neither did it stain lymph node lymphocytes that had been stimulated in culture with mitogens or alloantigens. The data suggest that M371 identifies a sessile population of IEL and that expression of the antigen is induced locally in the epithelium. In addition to IEL, M371 bound to some goblet cells in the mid and distal small gut but not in the proximal region. Double-staining experiments showed that M371 was highly specific for IEL with the phenotype Lyt-2+, Lyt-3-, Thy-1-, CD3+ and stained a majority of cells in this subpopulation. M371 precipitated a surface molecule approximately 275,000 MW in size, which was also precipitated by antibodies to CD45. Treatment of fixed IEL with sodium periodate prevented staining by M371, suggesting involvement of carbohydrate in the epitope. The specificity of M371 was shown to differ from that of the antibodies CT1 and CT2, which identify a carbohydrate determinant of CD45 expressed on cytotoxic lymphocytes and IEL. The possibility that the gut epithelium provides an environment for the functional differentiation of thymus-independent mucosal T cells is discussed.
Full text
PDF

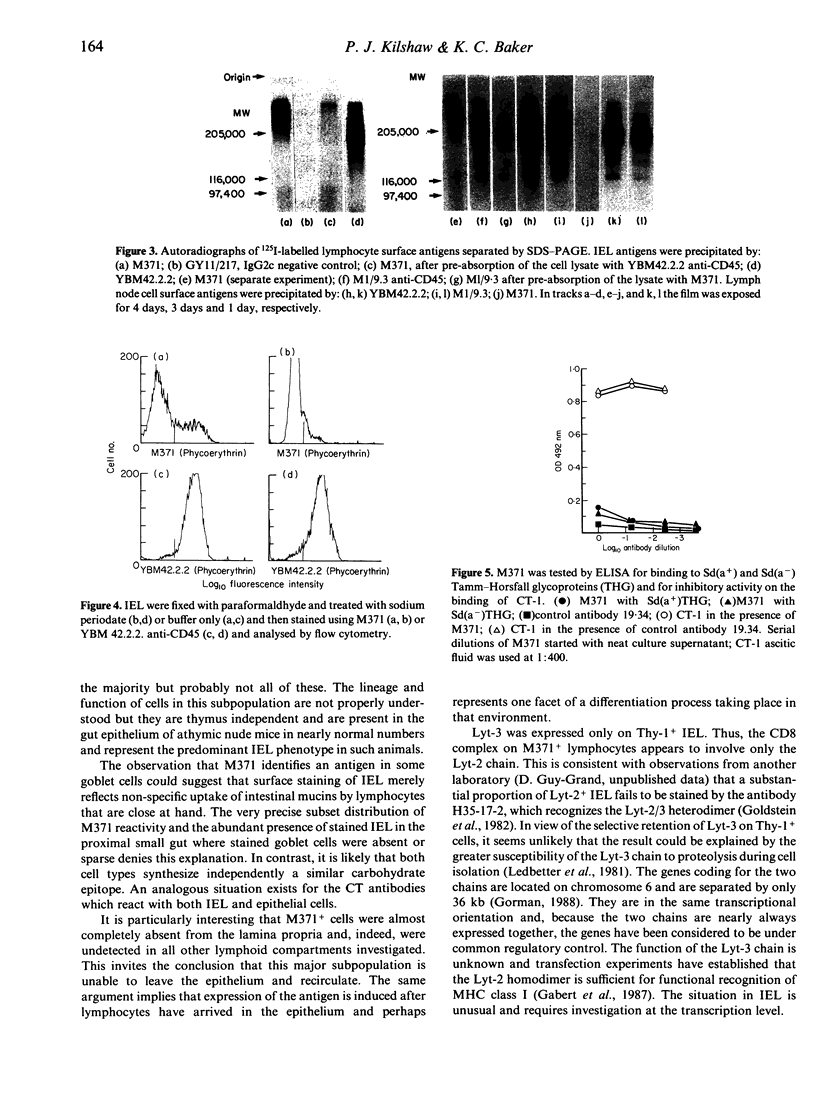


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. L., Cihak J., Lösch U., Cooper M. D. Avian T cells expressing gamma delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988 Oct 1;141(7):2200–2205. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Lisowska-Grospierre B., Griscelli C., Bhan A. K. A monoclonal antibody specific for rat intestinal lymphocytes. J Immunol. 1986 Jan;136(1):76–82. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Lefrancois L. Monoclonal antibodies specific for T cell-associated carbohydrate determinants react with human blood group antigens CAD and SDA. J Exp Med. 1988 Jan 1;167(1):119–131. doi: 10.1084/jem.167.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981 Jun;22(6):481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins W. O., 3rd Human intestinal intraepithelial lymphocytes. Gut. 1986 Aug;27(8):972–985. doi: 10.1136/gut.27.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald A. S., Yates A. D., Soh C. P., Morgan W. T., Watkins W. M. A blood group Sda-active pentasaccharide isolated from Tamm-Horsfall urinary glycoprotein. Biochem Biophys Res Commun. 1983 Sep 15;115(2):625–631. doi: 10.1016/s0006-291x(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Golstein P., Goridis C., Schmitt-Verhulst A. M., Hayot B., Pierres A., van Agthoven A., Kaufmann Y., Eshhar Z., Pierres M. Lymphoid cell surface interaction structures detected using cytolysis-inhibiting monoclonal antibodies. Immunol Rev. 1982;68:5–42. doi: 10.1111/j.1600-065x.1982.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Gorman S. D., Sun Y. H., Zamoyska R., Parnes J. R. Molecular linkage of the Ly-3 and Ly-2 genes. Requirement of Ly-2 for Ly-3 surface expression. J Immunol. 1988 May 15;140(10):3646–3653. [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilshaw P. J., Baker K. C. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol Lett. 1988 Jun;18(2):149–154. doi: 10.1016/0165-2478(88)90056-9. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Seaman W. E., Tsu T. T., Herzenberg L. A. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981 Jun 1;153(6):1503–1516. doi: 10.1084/jem.153.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L. Carbohydrate differentiation antigens of murine T cells: expression on intestinal lymphocytes and intestinal epithelium. J Immunol. 1987 May 15;138(10):3375–3384. [PubMed] [Google Scholar]
- Lefrancois L., Goodman T. Developmental sequence of T200 antigen modifications in murine T cells. J Immunol. 1987 Dec 1;139(11):3718–3724. [PubMed] [Google Scholar]
- Lefrançois L., Bevan M. J. Functional modifications of cytotoxic T-lymphocyte T200 glycoprotein recognized by monoclonal antibodies. Nature. 1985 Apr 4;314(6010):449–452. doi: 10.1038/314449a0. [DOI] [PubMed] [Google Scholar]
- Lefrançois L., Bevan M. J. Novel antigenic determinants of the T200 glycoprotein expressed preferentially by activated cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):374–383. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Whately R. J. Granular intraepithelial lymphocytes of the rat small intestine. I. Isolation, presence in T lymphocyte-deficient rats and bone marrow origin. Int Arch Allergy Appl Immunol. 1983;71(4):317–327. doi: 10.1159/000233414. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Lew A. M., Maloy W. L., Weston M. A., Bluestone J. A., Schwartz R. H., Coligan J. E., Kruisbeek A. M. Thymus-dependent and thymus-independent developmental pathways for peripheral T cell receptor-gamma delta-bearing lymphocytes. J Immunol. 1988 Jun 15;140(12):4091–4096. [PubMed] [Google Scholar]
- Parish C. R., Hogarth P. M., McKenzie I. F. Evidence that Thy-1 and Ly-5 (T-200) antigens interact with sulphated carbohydrates. Immunol Cell Biol. 1988 Jan;66(Pt 3):221–230. doi: 10.1038/icb.1988.28. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Thomas M. L., Lefrançois L. Differential expression of the leucocyte-common antigen family. Immunol Today. 1988 Oct;9(10):320–326. doi: 10.1016/0167-5699(88)91326-6. [DOI] [PubMed] [Google Scholar]
- Viney J. L., MacDonald T. T., Kilshaw P. J. T-cell receptor expression in intestinal intra-epithelial lymphocyte subpopulations of normal and athymic mice. Immunology. 1989 Apr;66(4):583–587. [PMC free article] [PubMed] [Google Scholar]
- Watt S. M., Gilmore D. J., Metcalf D., Cobbold S. P., Hoang T. K., Waldmann H. Segregation of mouse hemopoietic progenitor cells using the monoclonal antibody, YBM/42. J Cell Physiol. 1983 Apr;115(1):37–45. doi: 10.1002/jcp.1041150107. [DOI] [PubMed] [Google Scholar]