Abstract
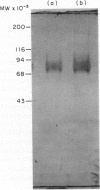
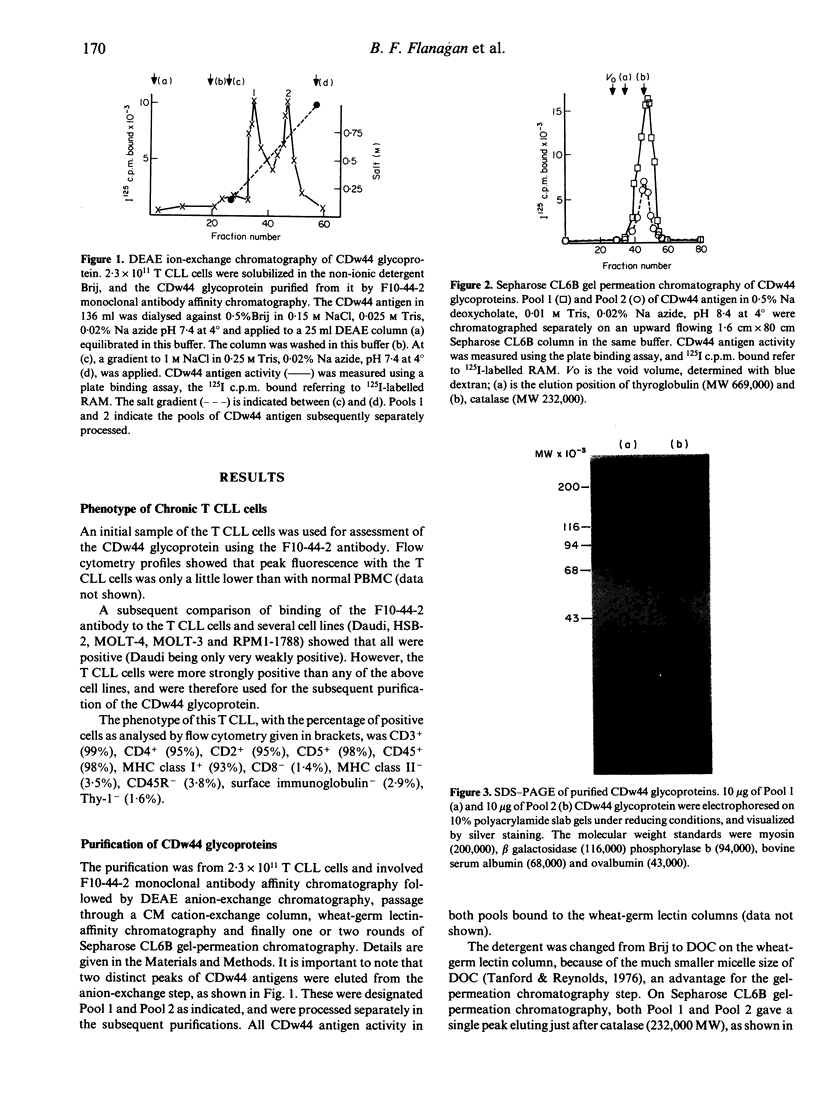
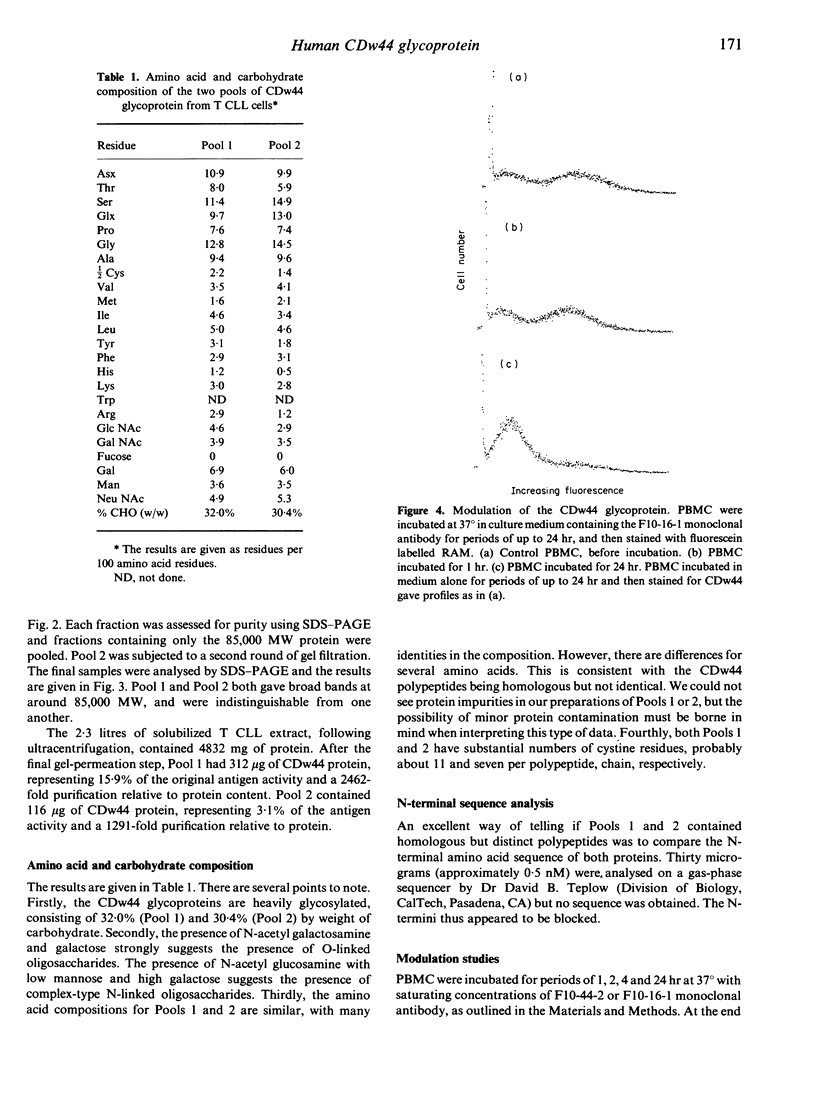
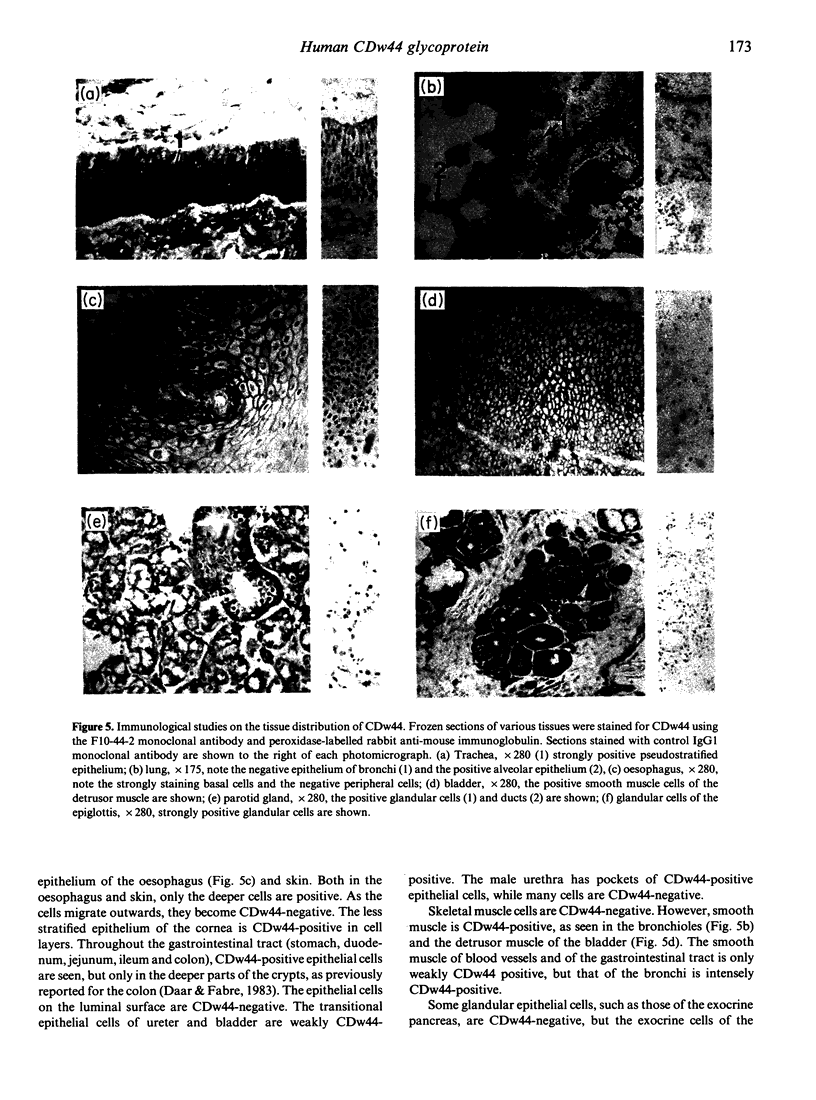
The CDw44 glycoprotein was purified from 2.3 x 10(11) CD3+ CD4+ CD8- T-chronic lymphocytic leukaemia (CLL) cells using F10-44-2 monoclonal antibody affinity chromatography, DEAE-Sepharose anion-exchange chromatography, passage down carboxymethyl (CM)-Sepharose cation-exchange columns, wheat germ lectin affinity chromatography and gel-permeation chromatography. On elution in non-ionic detergents from the DEAE column, two distinct peaks of antigen activity were obtained. The CDw44 glycoprotein in each peak was a glycoprotein of 85,000 MW, but the amino acid composition of the peaks was noticeably different. Carbohydrate compositions showed that each peak contained approximately 30% (w/w) carbohydrate, the composition suggesting both O-linked and complex N-linked glycans. Modulation studies with the F10-44-2 antibody on normal peripheral blood mononuclear cells (PBMC) demonstrated that the CDw44 glycoprotein of T cells consisted of one fraction that was readily modulated, and the other which was resistant to modulation. Detailed tissue distribution studies for CDw44 were performed using the F10-44-2 antibody on frozen sections of human tissues. CDw44 has a restricted tissue distribution, but is found on many highly diverse cell types (e.g. T lymphocytes, smooth muscle cells, some secretory glands, skin epithelial cells).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A. Purification of the glycoprotein lectin from the broad bean (Vicia faba) and a comparison of its properties with lectins of similar specificity. Biochem J. 1976 Apr 1;155(1):127–135. doi: 10.1042/bj1550127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A. The quantitation of glucosamine and galactosamine in glycoproteins after hydrolysis in p-toluenesulphonic acid. FEBS Lett. 1975 Dec 1;60(1):76–80. doi: 10.1016/0014-5793(75)80422-4. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Breder C. D., Dinarello C. A., Saper C. B. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988 Apr 15;240(4850):321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Jefferies W. A., Clark S. J., Chung L. P., Gordon S. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987 Aug 1;166(2):613–618. doi: 10.1084/jem.166.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. Demonstration with monoclonal antibodies of an unusual mononuclear cell infiltrate and loss of normal epithelial membrane antigens in human breast carcinomas. Lancet. 1981 Aug 29;2(8244):434–438. doi: 10.1016/s0140-6736(81)90773-x. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fabre J. W. The membrane antigens of human colorectal cancer cells: demonstration with monoclonal antibodies of heterogeneity within and between tumours and of anomalous expression of HLA-DR. Eur J Cancer Clin Oncol. 1983 Feb;19(2):209–220. doi: 10.1016/0277-5379(83)90419-4. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification with a monoclonal antibody of a predominantly B lymphocyte-specific determinant of the human leukocyte common antigen. Evidence for structural and possible functional diversity of the human leukocyte common molecule. J Exp Med. 1981 Apr 1;153(4):753–765. doi: 10.1084/jem.153.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Wiles M. V., Tunnacliffe A., Parkar M., Solomon E., Dalchau R., Fabre J. W. The gene, MIC4, which controls expression of the antigen defined by monoclonal antibody F10.44.2, is on human chromosome 11. Eur J Immunol. 1982 Aug;12(8):659–663. doi: 10.1002/eji.1830120807. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Haynes B. F., Harden E. A., Telen M. J., Hemler M. E., Strominger J. L., Palker T. J., Scearce R. M., Eisenbarth G. S. Differentiation of human T lymphocytes. I. Acquisition of a novel human cell surface protein (p80) during normal intrathymic T cell maturation. J Immunol. 1983 Sep;131(3):1195–1200. [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letarte M. Human p85 glycoprotein bears three distinct epitopes defined by several monoclonal antibodies. Mol Immunol. 1986 Jun;23(6):639–644. doi: 10.1016/0161-5890(86)90101-x. [DOI] [PubMed] [Google Scholar]
- Letarte M., Iturbe S., Quackenbush E. J. A glycoprotein of molecular weight 85,000 on human cells of B-lineage: detection with a family of monoclonal antibodies. Mol Immunol. 1985 Feb;22(2):113–124. doi: 10.1016/s0161-5890(85)80005-5. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McKenzie J. L., Dalchau R., Fabre J. W. Biochemical characterisation and localization in brain of a human brain-leucocyte membrane glycoprotein recognised by a monoclonal antibody. J Neurochem. 1982 Nov;39(5):1461–1466. doi: 10.1111/j.1471-4159.1982.tb12592.x. [DOI] [PubMed] [Google Scholar]
- McKenzie J. L., Fabre J. W. Human thy-1: unusual localization and possible functional significance in lymphoid tissues. J Immunol. 1981 Mar;126(3):843–850. [PubMed] [Google Scholar]
- Morstyn G., Metcalf D., Burgess A., Fabre J. W. Surface antigens on normal and leukaemic human cells detected by monoclonal antibodies. Scand J Haematol. 1981 Jan;26(1):19–30. doi: 10.1111/j.1600-0609.1981.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Quackenbush E. J., Letarte M. Identification of several cell surface proteins of non-T, non-B acute lymphoblastic leukemia by using monoclonal antibodies. J Immunol. 1985 Feb;134(2):1276–1285. [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring R., McMaster W. R., Sunderland C. A., Williams A. F. The predominant heavily glycosylated glycoproteins at the surface of rat lymphoid cells are differentiation antigens. Eur J Immunol. 1978 Dec;8(12):832–839. doi: 10.1002/eji.1830081203. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Telen M. J., Eisenbarth G. S., Haynes B. F. Human erythrocyte antigens. Regulation of expression of a novel erythrocyte surface antigen by the inhibitor Lutheran In(Lu) gene. J Clin Invest. 1983 Jun;71(6):1878–1886. doi: 10.1172/JCI110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telen M. J., Palker T. J., Haynes B. F. Human erythrocyte antigens: II. The In(Lu) gene regulates expression of an antigen on an 80-kilodalton protein of human erythrocytes. Blood. 1984 Sep;64(3):599–606. [PubMed] [Google Scholar]