Abstract
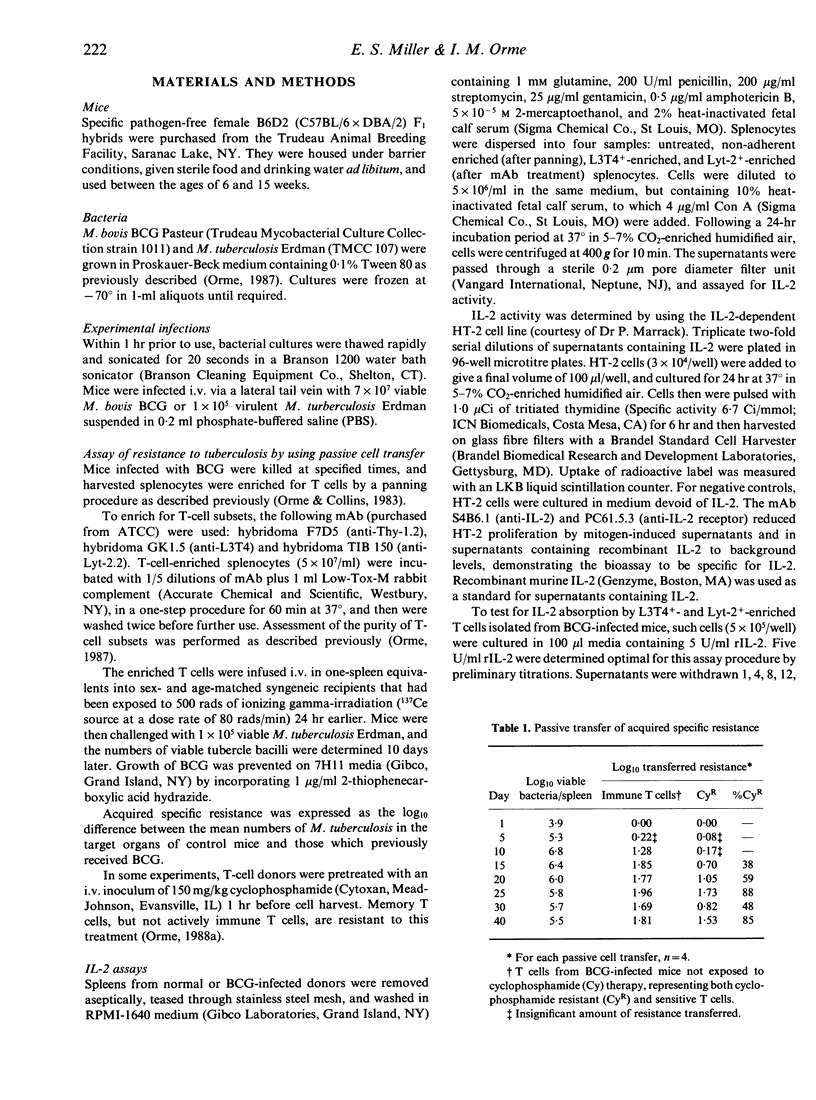
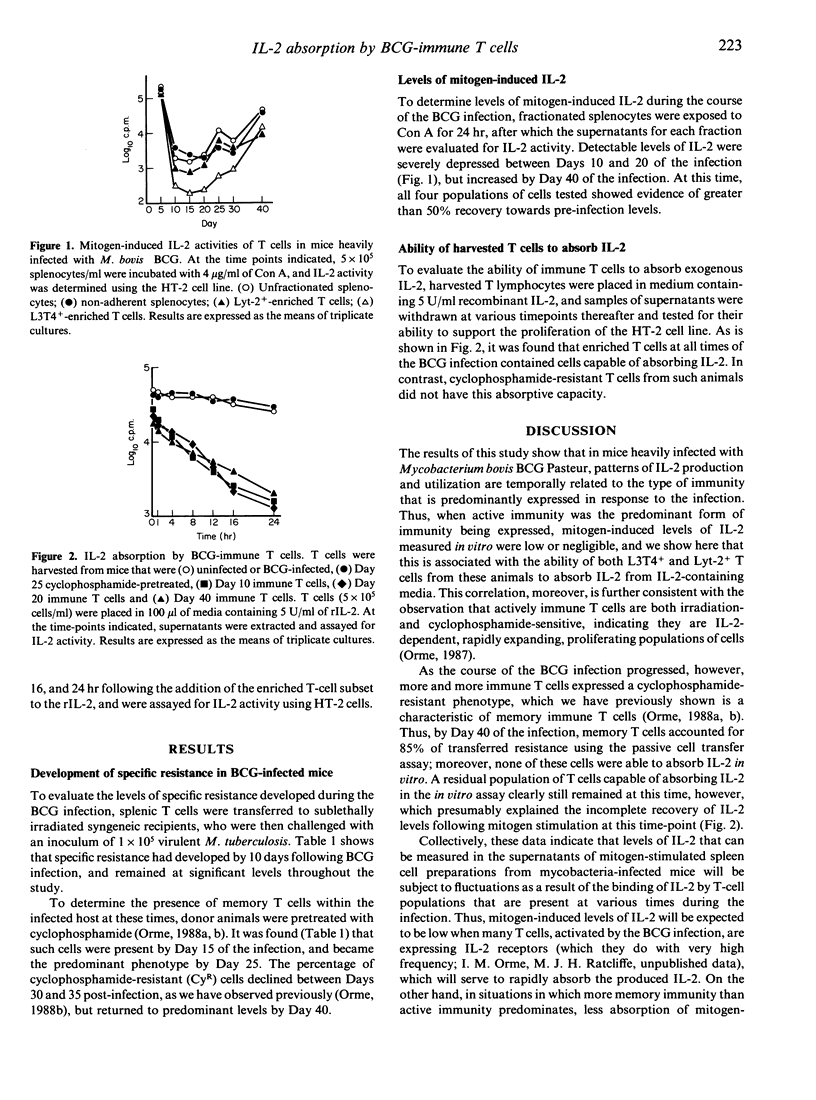
The results shown here demonstrate that in mice heavily infected with Mycobacterium bovis BCG Pasteur, mitogen-induced levels of interleukin-2 (IL-2) correlate temporally with the state of immunity that is being expressed in the animal during the course of the infection. Active immunity, which is conferred by populations of both CD4+ (L3T4) and CD8+ (Lyt-2) T lymphocytes, and memory immunity, which is mediated by a population of CD4+ T lymphocytes, were identified and distinguished in terms of their sensitivity to cyclophosphamide therapy, their ability to passively transfer specific resistance to infection with virulent Mycobacterium tuberculosis, and their capacity to produce and/or absorb IL-2. In this regard, concanavalin A (Con A)-stimulated L3T4+ and Lyt-2+-enriched splenocytes exhibited an apparent depression in measurable levels of IL-2 when harvested during the first 40 days of the infection, which could be explained by the subsequent observation that these T cells were capable of rapidly absorbing a known quantity of recombinant IL-2 in vitro. Detectable levels of IL-2 in these mitogen-stimulated supernatants began to rise after Day 25, which was temporally associated with a gradual shift from active immunity, to immunity mediated by cyclophosphamide-resistant memory T cells, which did not absorb IL-2 in vitro. These data indicate that fluctuations in apparent IL-2 production reflect changes in the type of immunity being expressed, rather than than some form of defect in IL-2 production.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrus L., Granelli-Piperno A., Reich E. Cytotoxic T cells both produce and respond to interleukin 2. J Exp Med. 1984 Feb 1;159(2):647–652. doi: 10.1084/jem.159.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Lymphocyte mediators and cellular hypersensitivity. N Engl J Med. 1973 Jan 18;288(3):143–149. doi: 10.1056/NEJM197301182880311. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Jeevan A., Asherson G. L. Recombinant interleukin-2 limits the replication of Mycobacterium lepraemurium and Mycobacterium bovis BCG in mice. Lymphokine Res. 1988 Summer;7(2):129–140. [PubMed] [Google Scholar]
- Kendall L., Sabbadini E. Effect of Bacillus Calmette-Guérin on the in vitro generation of cytotoxic T lymphocytes. I. Effect of BCG on the frequency of cytotoxic T lymphocyte precursors and on the production of helper factors. J Immunol. 1981 Jul;127(1):234–238. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Evidence for a biphasic memory T-cell response to high dose BCG vaccination in mice. Tubercle. 1988 Jun;69(2):125–131. doi: 10.1016/0041-3879(88)90075-x. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Ratcliffe M. J., Collins F. M. Acquired immunity to heavy infection with Mycobacterium bovis bacillus Calmette-Guérin and its relationship to the development of nonspecific unresponsiveness in vitro. Cell Immunol. 1984 Oct 15;88(2):285–296. doi: 10.1016/0008-8749(84)90162-x. [DOI] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Hatakeyama M., Kashima N., Fuse A., Hamuro J., Nishi-Takaoka C., Yamada G. Molecular analysis of the interleukin-2 system. Immunol Rev. 1986 Aug;92:121–133. doi: 10.1111/j.1600-065x.1986.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Legault D. Mechanisms underlying the depressed production of interleukin-2 in spleen and lymph node cell cultures of mice infected with Mycobacterium bovis BCG. Infect Immun. 1986 Mar;51(3):826–831. doi: 10.1128/iai.51.3.826-831.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Lemieux S. Mechanisms of action of Mycobacterium bovis BCG-induced suppressor cells in mitogen-induced blastogenesis. Infect Immun. 1982 Apr;36(1):263–270. doi: 10.1128/iai.36.1.263-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



