Abstract
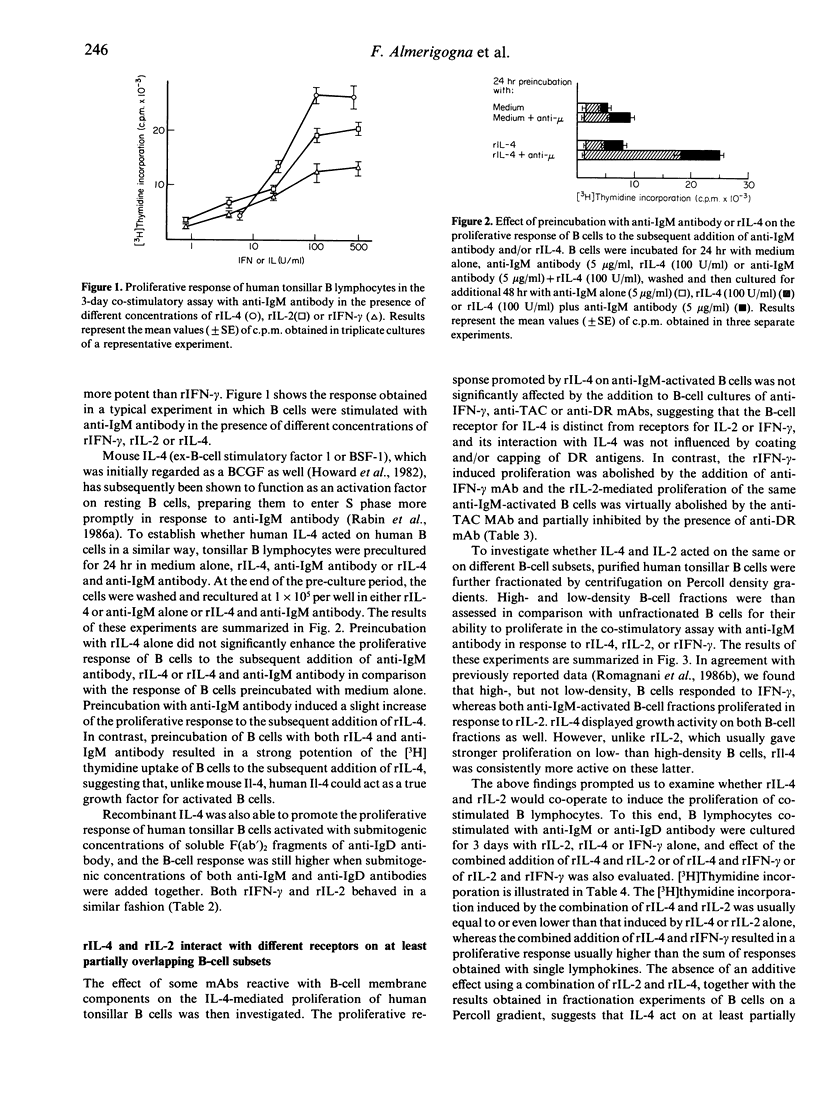
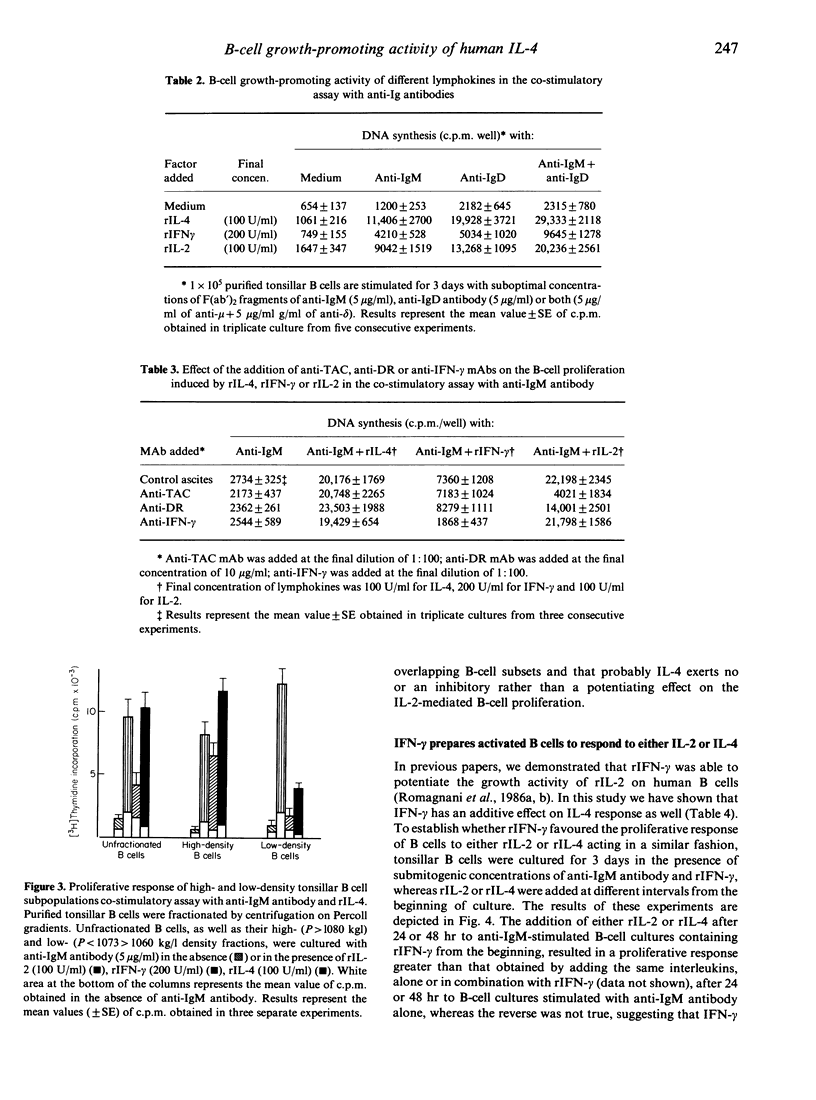
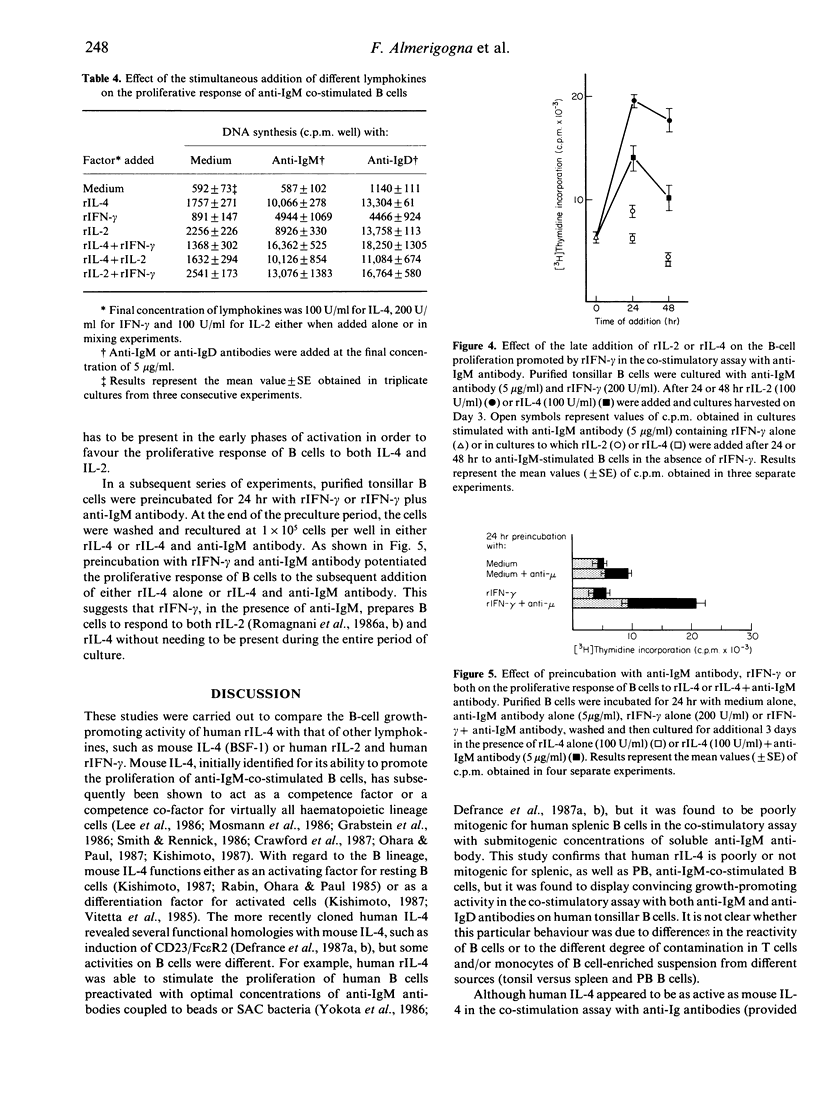
Human recombinant interleukin-4 (rIL-4) was assessed for its ability to promote the proliferative response of purified human B cells co-stimulated with submitogenic concentrations of soluble F(ab')2 fragments of anti-immunoglobulin (Ig) antibodies. The growth-promoting activity of rIL-4 was usually as potent as, or even more potent than, that of recombinant interleukin-2 (rIL-2), and more potent than that of recombinant interferon-gamma (rIFN-gamma). Preincubation with rIL-4 did not cause enhancement of the proliferative response of B cells to the subsequent addition of rIL-4 and anti-IgM antibody. In contrast, the proliferative response of B cells preincubated with anti-IgM antibody and rIL-4 was potentiated by the subsequent addition of rIL-4. The simultaneous addition of rIFN-gamma and rIL-2 or rIFN-gamma and rIL-4 had an additive effect in comparison with the response induced by rIL-2 or rIL-4 alone, respectively, whereas simultaneous addition of rIL-2 and rIL-4 induced a response equal or lower than that stimulated by rIL-2 or rIL-4 alone. The addition of rIFN-gamma at the beginning of culture or preincubation of B cells with rIFN-gamma and anti-IgM antibody potentiated the proliferative response of B cells to the subsequent addition of either rIL-2 or rIL-4. Taken together, these data suggest that rIL-4 acts as a growth factor for activated human B cells and displays on such cells a growth-promoting activity similar to that of rIL-2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almerigogna F., Biagiotti R., Giudizi G. M., Del Prete G. F., Maggi E., Mazzetti M., Alessi A., Ricci M., Romagnani S. Different reactivity of activated human B cells to B-cell growth factor and interleukin 2 in the costimulation assay with anti-IgM antibody and in the preactivation assay with Staphylococcus bacteria. Cell Immunol. 1985 Oct 15;95(2):358–367. doi: 10.1016/0008-8749(85)90323-5. [DOI] [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Vanbervliet B., Banchereau J. Human interferon-gamma acts as a B cell growth factor in the anti-IgM antibody co-stimulatory assay but has no direct B cell differentiation activity. J Immunol. 1986 Dec 15;137(12):3861–3867. [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Giudizi M. G., Biagiotti R., Almerigogna F., Alessi A., Tiri A., Del Prete G. F., Ferrone S., Romagnani S. Role of HLA class I and class II antigens in activation and differentiation of B cells. Cell Immunol. 1987 Aug;108(1):97–108. doi: 10.1016/0008-8749(87)90196-1. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Eisenman J., Mochizuki D., Shanebeck K., Conlon P., Hopp T., March C., Gillis S. Purification to homogeneity of B cell stimulating factor. A molecule that stimulates proliferation of multiple lymphokine-dependent cell lines. J Exp Med. 1986 Jun 1;163(6):1405–1414. doi: 10.1084/jem.163.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. B-cell stimulatory factors (BSFs): molecular structure, biological function, and regulation of expression. J Clin Immunol. 1987 Sep;7(5):343–355. doi: 10.1007/BF00917012. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Ostberg L., Stegagno M., Kimura A. K., Orn A., Sjöberg O. A rapid method for the separation of functional lymphoid cell populations of human and animal origin on PVP-silica (Percoll) density gradients. Scand J Immunol. 1979;10(6):563–573. doi: 10.1111/j.1365-3083.1979.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingari M. C., Gerosa F., Carra G., Accolla R. S., Moretta A., Zubler R. H., Waldmann T. A., Moretta L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature. 1984 Dec 13;312(5995):641–643. doi: 10.1038/312641a0. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Finkelman F. D., Sarma C., Ohara J., Serrate S. Recombinant interferon-gamma inhibits the B cell proliferative response stimulated by soluble but not by Sepharose-bound anti-immunoglobulin antibody. J Immunol. 1985 Oct;135(4):2513–2517. [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Longo D. L., Volkman D. J., Smith K. A., Fauci A. S. Interleukin 2 receptors on human B cells. Implications for the role of interleukin 2 in human B cell function. J Exp Med. 1985 Jan 1;161(1):181–197. doi: 10.1084/jem.161.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Receptors for B-cell stimulatory factor-1 expressed on cells of haematopoietic lineage. Nature. 1987 Feb 5;325(6104):537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- Rabin E. M., Mond J. J., Ohara J., Paul W. E. B cell stimulatory factor 1 (BSF-1) prepares resting B cells to enter S phase in response to anti-IgM and lipopolysaccharide. J Exp Med. 1986 Aug 1;164(2):517–531. doi: 10.1084/jem.164.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. M., Mond J. J., Ohara J., Paul W. E. Interferon-gamma inhibits the action of B cell stimulatory factor (BSF)-1 on resting B cells. J Immunol. 1986 Sep 1;137(5):1573–1576. [PubMed] [Google Scholar]
- Romagnani S., Giudizi G. M., Almerigogna F., Biagiotti R., Alessi A., Mingari C., Liang C. M., Moretta L., Ricci M. Analysis of the role of interferon-gamma, interleukin 2 and a third factor distinct from interferon-gamma and interleukin 2 in human B cell proliferation. Evidence that they act at different times after B cell activation. Eur J Immunol. 1986 Jun;16(6):623–629. doi: 10.1002/eji.1830160607. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi G. M., Maggi E., Almerigogna F., Biagiotti R., Del Prete G., Mazzetti M., Alessi A., Vercelli D., Ricci M. Synergy of B cell growth factor and interleukin 2 in the proliferation of activated human B cells. Eur J Immunol. 1985 Dec;15(12):1158–1164. doi: 10.1002/eji.1830151203. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Almerigogna F., Ricci M. Interaction of staphylococcal protein A with membrane components of IgM- and/or IgD-bearing lymphocytes from human tonsil. J Immunol. 1980 Apr;124(4):1620–1626. [PubMed] [Google Scholar]
- Sharma S., Mehta S., Morgan J., Maizel A. Molecular cloning and expression of a human B-cell growth factor gene in Escherichia coli. Science. 1987 Mar 20;235(4795):1489–1492. doi: 10.1126/science.3547651. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Uchino H. Expression of Tac antigen on activated normal human B cells. J Exp Med. 1984 Aug 1;160(2):612–617. doi: 10.1084/jem.160.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]


